Plasmacytoid urothelial carcinoma: response to chemotherapy and oncologic outcomes
- PMID: 34109262
- PMCID: PMC8186525
- DOI: 10.3233/blc-190258
Plasmacytoid urothelial carcinoma: response to chemotherapy and oncologic outcomes
Abstract
Background: Plasmacytoid urothelial carcinoma is a rare bladder cancer variant with scarce data on outcomes and prognostic factors.
Objective: We report our institutional experience with this histology to determine response to neoadjuvant chemotherapy, definitive surgery and survival.
Methods: We conducted a retrospective chart review of consecutive patients with plasmacytoid, as well as conventional urothelial carcinoma (for comparison) seen in our institution (2007 - 2018). Baseline characteristics, clinicopathologic and treatment data were captured. T-test, chi-squared and log-rank test was used for group comparison. Kaplan Meier method was used for estimation of overall survival and Cox regression for identification of prognostic factors.
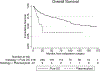
Results: 64 patients with plasmacytoid and 418 with conventional urothelial histology were identified; 53% of those with plasmacytoid presented with cT3/4 stage and 67% underwent extirpative surgery. Patients with plasmacytoid histology had higher rates of pT3/4 (65% vs. 28%), nodal disease (37% vs. 16%) and positive surgical margins (23% vs. 5%) compared to urothelial group (p < 0.01), as well as higher incidence of post-operative recurrence (47% vs. 29%, p = 0.05) and lower ypT0N0 rates after neoadjuvant chemotherapy (10% vs. 33%, p = 0.03). Plasmacytoid histology was associated with lower median overall survival compared to conventional urothelial (24 vs. 154 months, p < 0.01).
Conclusions: Plasmacytoid urothelial carcinoma frequently presented with advanced stage at diagnosis and extirpative surgery, poor pathologic response to neoadjuvant chemotherapy, and inferior outcomes, when compared to conventional urothelial. Prospective trials evaluating upfront cystectomy versus preoperative chemotherapy and/or novel treatments should be considered.
Keywords: cystectomy; neoadjuvant therapy; transitional cell carcinoma; urinary bladder neoplasms.
Conflict of interest statement
Conflict of Interest Statement Leonidas Nikolaos Diamantopoulos No Relationships to Disclose Ali Raza Khaki Stock and Other Ownership Interests - Pfizer; Procter & Gamble; Walgreens Boots Alliance Petros Grivas: personal fees and other from Genentech, personal fees and other from Bayer, personal fees and other from Merck & Co., personal fees and other from Mirati Therapeutics, other from Oncogenex, personal fees and other from Pfizer, personal fees and other from Bristol-Myers Squibb, personal fees, non-financial support and other from Astra Zeneca, personal fees from Biocept, personal fees, non-financial support and other from ClovisOncology, personal fees from EMD Serono, personal fees from Seattle Genetics, personal fees from Foundation Medicine, personal fees from Driver Inc., personal fees from QED Therapeutics, personal fees from Heron Therapeutics, personal fees from Janssen, other from Bavarian Nordic, other from Immunomedics, other from Debiopharm, personal fees from GlaxoSmithKline, personal fees from Roche, personal fees from Genzyme, personal fees from Exelixis. John L. Gore Research Funding – Ferring Pharmaceuticals George R Schade Patents, Royalties, Other Intellectual Property - Global Cancer Technology Andrew Caleb Hsieh Honoraria - Hotspot Therapeutics Research Funding - eFFECTOR Inc Patents, Royalties, Other Intellectual Property - MTOR modulators and uses thereof Patent number: 9629843; USE OF TRANSLATIONAL PROFILING TO IDENTIFY TARGET MOLECULES FOR THERAPEUTIC TREATMENT Publication number: 20140288097 John Kyung Lee Research Funding - Immunomedics Todd Yezefski No Relationships to Disclose Evan Y. Yu Consulting or Advisory Role - Amgen; AstraZeneca; Bayer; Churchill Pharmaceuticals; Dendreon; EMD Serono; Incyte; Janssen; Merck; QED; Seattle Genetics; Tolmar Research Funding - Agensys; Astellas Pharma; Bayer; Dendreon; Genentech/Roche; Merck; Seattle Genetics Michael Thomas Schweizer Consulting or Advisory Role - Janssen Research Funding - AstraZeneca; Janssen; Madison Vaccines, Inc.; Pfizer; Roche; Zenith Epigenetics Heather H. Cheng Research Funding - Astellas Medivation; Clovis Oncology; Color Foundation; Inovio Pharmaceuticals; Janssen; Sanofi Sarah P. Psutka Honoraria - Prime Education Travel, Accommodations, Expenses - Prime Education Daniel W. Lin Consulting or Advisory Role - Astellas Pharma; Clovis Oncology; Dendreon Research Funding - GenomeDx; Genomic Health; MDxHealth Maria S. Tretiakova No Relationships to Disclose Funda Vakar-Lopez No Relationships to Disclose Robert B. Montgomery Research Funding - AstraZeneca; ESSA; Ferring; Janssen Oncology Jonathan L. Wright Speakers’ Bureau - Foundation Health Partners Carroll Cancer Center; OncLive; Seattle Cancer Care Alliance Research Funding - Altor BioScience; Merck; Nucleix Other Relationship - Movember; UpToDate
Figures

References
-
- Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. European urology. 2016;70(1):106–19. - PubMed
-
- Fox MD, Xiao L, Zhang M, Kamat AM, Siefker-Radtke A, Zhang L, Dinney CP, Czerniak B, Guo CC. Plasmacytoid Urothelial Carcinoma of the Urinary Bladder: A Clinicopathologic and Immunohistochemical Analysis of 49 Cases. American journal of clinical pathology. 2017;147(5):500–6. - PubMed
-
- Keck B, Stoehr R, Wach S, Rogler A, Hofstaedter F, Lehmann J, Montironi R, Sibonye M, Fritsche HM, Lopez-Beltran A, Epstein JI, Wullich B, Hartmann A. The plasmacytoid carcinoma of the bladder--rare variant of aggressive urothelial carcinoma. International journal of cancer. 2011;129(2):346–54. - PubMed
-
- Cockerill PA, Cheville JC, Boorjian SA, Blackburne A, Thapa P, Tarrell RF, Frank I. Outcomes Following Radical Cystectomy for Plasmacytoid Urothelial Carcinoma: Defining the Need for Improved Local Cancer Control. Urology. 2017;102:143–7. - PubMed
-
- Kaimakliotis HZ, Monn MF, Cary KC, Pedrosa JA, Rice K, Masterson TA, Gardner TA, Hahn NM, Foster RS, Bihrle R, Cheng L, Koch MO. Plasmacytoid variant urothelial bladder cancer: is it time to update the treatment paradigm? Urologic oncology. 2014;32(6):833–8. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

