Comparative ecology of Guinea baboons (Papio papio)
- PMID: 34109265
- PMCID: PMC8182668
- DOI: 10.5194/pb-8-19-2021
Comparative ecology of Guinea baboons (Papio papio)
Abstract
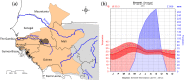
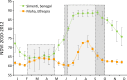
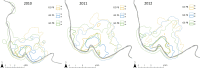
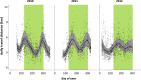
Thorough knowledge of the ecology of a species or population is an essential prerequisite for understanding the impact of ecology on the evolution of their respective social systems. Because of their diversity of social organizations, baboons (Papio spp.) are a useful model for comparative studies. Comparative ecological information was missing for Guinea baboons (Papio papio), however. Here we provide data on the ecology of Guinea baboons in a comparative analysis on two geographical scales. First, we compare climate variables and land cover among areas of occurrence of all six baboon species. Second, we describe home range size, habitat use, ranging behaviour, and diet from a local population of Guinea baboons ranging near the Centre de Recherche de Primatologie (CRP) Simenti in the Niokolo-Koba National Park, Senegal. Home ranges and daily travel distances at Simenti varied seasonally, yet the seasonal patterns in their daily travel distance did not follow a simple dry vs. rainy season pattern. Chemical food composition falls within the range of other baboon species. Compared to other baboon species, areas occupied by Guinea baboons experience the highest variation in precipitation and the highest seasonality in precipitation. Although the Guinea baboons' multi-level social organization is superficially similar to that of hamadryas baboons (P. hamadryas), the ecologies of the two species differ markedly. Most Guinea baboon populations, including the one at Simenti, live in more productive habitats than hamadryas baboons. This difference in the ecology of the two species contradicts a simple evolutionary relation between ecology and social system and suggests that other factors have played an additional role here.
Copyright: © 2021 Dietmar Zinner et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






Similar articles
-
Charting the neglected West: The social system of Guinea baboons.Am J Phys Anthropol. 2017 Jan;162 Suppl 63(Suppl Suppl 63):15-31. doi: 10.1002/ajpa.23144. Am J Phys Anthropol. 2017. PMID: 28105722 Free PMC article.
-
Group Composition of Guinea Baboons (Papio papio) at a Water Place Suggests a Fluid Social Organization.Int J Primatol. 2011 Jun;32(3):652-668. doi: 10.1007/s10764-011-9493-z. Epub 2011 Feb 11. Int J Primatol. 2011. PMID: 21654901 Free PMC article.
-
Coordination during group departures and progressions in the tolerant multi-level society of wild Guinea baboons (Papio papio).Sci Rep. 2021 Nov 9;11(1):21938. doi: 10.1038/s41598-021-01356-6. Sci Rep. 2021. PMID: 34754018 Free PMC article.
-
A proper study for mankind: Analogies from the Papionin monkeys and their implications for human evolution.Am J Phys Anthropol. 2001;Suppl 33:177-204. doi: 10.1002/ajpa.10021. Am J Phys Anthropol. 2001. PMID: 11786995 Review.
-
Confessions of a baboon watcher: from inside to outside the paradigm.Primates. 2023 Jul;64(4):393-406. doi: 10.1007/s10329-023-01060-1. Epub 2023 May 10. Primates. 2023. PMID: 37165179 Free PMC article. Review.
Cited by
-
Impact of food availability and predator presence on patterns of landscape partitioning among neighbouring Guinea baboon (Papio papio) parties.Mov Ecol. 2025 Feb 22;13(1):9. doi: 10.1186/s40462-025-00534-9. Mov Ecol. 2025. PMID: 39987137 Free PMC article.
-
Age- and size-related changes in hind limb muscles in two baboon species (Papio anubis and P. papio).J Anat. 2025 Jan;246(1):108-119. doi: 10.1111/joa.14140. Epub 2024 Sep 23. J Anat. 2025. PMID: 39313987 Free PMC article.
-
Identifying late Pleistocene and Holocene refugia for baboons.Commun Biol. 2025 Jul 4;8(1):1003. doi: 10.1038/s42003-025-08419-8. Commun Biol. 2025. PMID: 40615677 Free PMC article.
-
A Comprehensive Overview of Baboon Phylogenetic History.Genes (Basel). 2023 Feb 28;14(3):614. doi: 10.3390/genes14030614. Genes (Basel). 2023. PMID: 36980887 Free PMC article. Review.
-
Human and macaque pairs employ different coordination strategies in a transparent decision game.Elife. 2023 Jan 12;12:e81641. doi: 10.7554/eLife.81641. Elife. 2023. PMID: 36633125 Free PMC article.
References
-
- Adam JG. Le milieu biologique. Flore et végétation. In: Dupuy AR, editor. Le Niokolo Koba. Premier grand Parc national de la République du Sénégal. Grand Imprimerie Africaine; Dakar, Senegal: 1971. pp. 43–64.
-
- Alberts SC, Hollister-Smith JA, Mututua RS, Sayialel SN, Muruthi PM, Warutere JK, Altmann J. Seasonality and long term change in a savanna environment. In: Brockman DK, van Schaik CP, editors. Seasonality in Primates Studies of Living and Extinct Human and Non-Human Primates. Cambridge University Press; Cambridge, UK: 2005. pp. 157–196.
-
- Altmann J. Primate males go where females are. Anim Behav. 1990;39:193–195. doi: 10.1016/S0003-3472(05)80740-7. - DOI
-
- Altmann SA. Foraging for Survival. Yearling Baboons in Africa. The University of Chicago Press; Chicago, USA: 1998.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
