Functional Connectivity of Vermis Correlates with Future Gait Impairments in Parkinson's Disease
- PMID: 34109682
- PMCID: PMC8595492
- DOI: 10.1002/mds.28684
Functional Connectivity of Vermis Correlates with Future Gait Impairments in Parkinson's Disease
Abstract
Background: Dysfunction of cerebellar vermis contributes to gait abnormalities in multiple conditions and may play a key role in gait impairment in Parkinson's disease (PD).
Objective: The purpose of this study was to investigate whether altered resting-state functional connectivity of the vermis relates to subsequent impairment of specific domains of gait in PD.
Methods: We conducted morphometric and resting-state functional connectivity MRI analyses contrasting 45 PD and 32 age-matched healthy participants. Quantitative gait measures were acquired with a GAITRite walkway at varying intervals after functional connectivity data acquisition.
Results: At baseline, PD participants had significantly altered functional connectivity between vermis and sensorimotor cortex compared with controls. Altered vermal functional connectivity with bilateral paracentral lobules correlated with subsequent measures of variability in stride length, step time, and single support time after controlling for confounding variables including the interval between imaging and gait measures. Similarly, altered functional connectivity between vermis and left sensorimotor cortex correlated with mean stride length and its variability. Vermis volume did not relate to any gait measure. PD participants did not differ from controls in vermis volume or cortical thickness at the site of significant regional clusters. Only altered lobule V:sensorimotor cortex functional connectivity correlated with subsequent gait measures in exploratory analyses involving all the other cerebellar lobules.
Conclusions: These results demonstrate that abnormal vermal functional connectivity with sensorimotor cortex, in the absence of relevant vermal or cortical atrophy, correlates with subsequent gait impairment in PD. Our data reflect the potential of vermal functional connectivity as a novel imaging biomarker of gait impairment in PD. © 2021 International Parkinson and Movement Disorder Society.
Keywords: Parkinson disease; cerebellum; functional connectivity; gait impairment; vermis.
© 2021 International Parkinson and Movement Disorder Society.
Figures

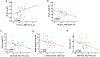
References
-
- Gilat M, Bell PT, Ehgoetz Martens KA, et al. Dopamine depletion impairs gait automaticity by altering cortico-striatal and cerebellar processing in Parkinson’s disease. NeuroImage 2017;152:207–220. - PubMed
Publication types
MeSH terms
Grants and funding
- U19 NS110456/NS/NINDS NIH HHS/United States
- U01 NS113851/NS/NINDS NIH HHS/United States
- U54 NS116025/NS/NINDS NIH HHS/United States
- R33 AT010753/AT/NCCIH NIH HHS/United States
- RF1 AG064937/AG/NIA NIH HHS/United States
- R01 AG050263/AG/NIA NIH HHS/United States
- R01 NS075321/NS/NINDS NIH HHS/United States
- R01 NS075527/NS/NINDS NIH HHS/United States
- R01 NS097437/NS/NINDS NIH HHS/United States
- R01 NS097799/NS/NINDS NIH HHS/United States
- R21 AG063974/AG/NIA NIH HHS/United States
- RF1 NS075321/NS/NINDS NIH HHS/United States
- R01 NS092865/NS/NINDS NIH HHS/United States
- R01 NS109487/NS/NINDS NIH HHS/United States
- R01 NS107281/NS/NINDS NIH HHS/United States
- R01 NS103957/NS/NINDS NIH HHS/United States
- P30 NS098577/NS/NINDS NIH HHS/United States
- R01 HD092444/HD/NICHD NIH HHS/United States
- U54 TR001456/TR/NCATS NIH HHS/United States
- KL2 TR002346/TR/NCATS NIH HHS/United States
- R01 ES029524/ES/NIEHS NIH HHS/United States
- U54 NS065701/NS/NINDS NIH HHS/United States
- R61 AT010753/AT/NCCIH NIH HHS/United States
- U10 NS077384/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

