Efficacy and safety of esaxerenone (CS-3150) in Japanese patients with type 2 diabetes and macroalbuminuria: a multicenter, single-arm, open-label phase III study
- PMID: 34110524
- PMCID: PMC8421271
- DOI: 10.1007/s10157-021-02075-y
Efficacy and safety of esaxerenone (CS-3150) in Japanese patients with type 2 diabetes and macroalbuminuria: a multicenter, single-arm, open-label phase III study
Abstract
Background: Esaxerenone has potential renoprotective effects and reduces the urinary albumin-to-creatinine ratio (UACR) in patients with diabetic kidney disease and overt nephropathy. We investigated the efficacy and safety of esaxerenone in Japanese patients with type 2 diabetes (T2D) and macroalbuminuria (UACR ≥ 300 mg/g creatinine).
Methods: We conducted a multicenter, single-arm, open-label phase III study in 56 patients with T2D and UACR ≥ 300 mg/g creatinine with estimated glomerular filtration rate (eGFR) ≥ 30 mL/min/1.73 m2 and treated with a renin-angiotensin system inhibitor. Patients received esaxerenone for 28 weeks at 1.25 mg/day initially with titration to 2.5 mg/day based on serum potassium (K+) monitoring. Efficacy was evaluated as the change in UACR from baseline to week 28. Safety endpoints included adverse events (AEs), incidence of serum K+ increase, and change in eGFR from baseline.
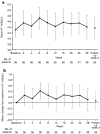
Results: UACR decreased by 54.6% (95% CI 46.9%, 61.3%) on average from baseline (544.1 mg/g creatinine) to the end of treatment (246.8 mg/g creatinine); 51.8% of patients showed improvement to early nephropathy. AE incidence was 69.6%. Three patients (5.4%) had serum K+ levels ≥ 6.0 mEq/L or ≥ 5.5 mEq/L on two consecutive occasions. Hyperkalemia in two patients was transient and resolved during the treatment period. One patient discontinued following two consecutive serum K+ values ≥ 5.5 mEq/L. The maximum change from baseline in eGFR was - 8.3 mL/min/1.73 m2 at week 24.
Conclusions: Esaxerenone reduced UACR in Japanese patients with T2D and UACR ≥ 300 mg/g creatinine; more than half experienced a transition from UACR ≥ 300 mg/g creatinine to UACR < 300 mg/g creatinine.
Clinical trial registration: JapicCTI-173696.
Keywords: Esaxerenone; Macroalbuminuria; Phase III; Type 2 diabetes; Urinary albumin-to-creatinine ratio.
© 2021. The Author(s).
Conflict of interest statement
Employment: Yasuyuki Okuda (Daiichi Sankyo Co., Ltd.) and Tomoko Sawanobori (Daiichi Sankyo Co., Ltd); Personal fees: Sadayoshi Ito (Daiichi Sankyo Co., Ltd.), Naoki Kashihara (Daiichi Sankyo Co., Ltd.), Kenichi Shikata (Daiichi Sankyo Co., Ltd.), Masaomi Nangaku (Daiichi Sankyo Co., Ltd.), and Takashi Wada (Daiichi Sankyo Co., Ltd); Grants/research funding received: Sadayoshi Ito (Daiichi Sankyo Co., Ltd.), Naoki Kashihara (Daiichi Sankyo Co., Ltd.), and Masaomi Nangaku (Daiichi Sankyo Co., Ltd).
Figures



Similar articles
-
Esaxerenone (CS-3150) in Patients with Type 2 Diabetes and Microalbuminuria (ESAX-DN): Phase 3 Randomized Controlled Clinical Trial.Clin J Am Soc Nephrol. 2020 Dec 7;15(12):1715-1727. doi: 10.2215/CJN.06870520. Epub 2020 Nov 25. Clin J Am Soc Nephrol. 2020. PMID: 33239409 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Esaxerenone (CS-3150) for the Treatment of Type 2 Diabetes with Microalbuminuria: A Randomized, Double-Blind, Placebo-Controlled, Phase II Trial.Clin J Am Soc Nephrol. 2019 Aug 7;14(8):1161-1172. doi: 10.2215/CJN.14751218. Epub 2019 Jun 27. Clin J Am Soc Nephrol. 2019. PMID: 31248950 Free PMC article. Clinical Trial.
-
Synergistic reduction in albuminuria in type 2 diabetic mice by esaxerenone (CS-3150), a novel nonsteroidal selective mineralocorticoid receptor blocker, combined with an angiotensin II receptor blocker.Hypertens Res. 2020 Nov;43(11):1204-1213. doi: 10.1038/s41440-020-0495-0. Epub 2020 Jul 2. Hypertens Res. 2020. PMID: 32616846 Free PMC article.
-
Spironolactone Add-on for Preventing or Slowing the Progression of Diabetic Nephropathy: A Meta-analysis.Clin Ther. 2015 Sep;37(9):2086-2103.e10. doi: 10.1016/j.clinthera.2015.05.508. Epub 2015 Aug 5. Clin Ther. 2015. PMID: 26254276 Review.
-
Management of hyperkalemia during treatment with mineralocorticoid receptor blockers: findings from esaxerenone.Hypertens Res. 2021 Apr;44(4):371-385. doi: 10.1038/s41440-020-00569-y. Epub 2020 Nov 20. Hypertens Res. 2021. PMID: 33214722 Free PMC article. Review.
Cited by
-
Nighttime home blood pressure lowering effect of esaxerenone in patients with uncontrolled nocturnal hypertension: the EARLY-NH study.Hypertens Res. 2023 Jul;46(7):1782-1794. doi: 10.1038/s41440-023-01292-0. Epub 2023 May 12. Hypertens Res. 2023. PMID: 37173430 Free PMC article.
-
Mineralocorticoid Receptor Antagonists for Preventing Chronic Kidney Disease Progression: Current Evidence and Future Challenges.Int J Mol Sci. 2023 Apr 23;24(9):7719. doi: 10.3390/ijms24097719. Int J Mol Sci. 2023. PMID: 37175424 Free PMC article. Review.
-
Safety of esaxerenone (CS-3150) and its impacts on blood pressure and renal function: A systematic review and meta-analysis.Medicine (Baltimore). 2025 Aug 1;104(31):e43615. doi: 10.1097/MD.0000000000043615. Medicine (Baltimore). 2025. PMID: 40760566 Free PMC article.
-
Nonsteroidal Mineralocorticoid Receptor Antagonist Eliciting Cardiorenal Protection Is a New Option for Patients with Chronic Kidney Disease.Kidney Dis (Basel). 2022 Nov 14;9(1):12-25. doi: 10.1159/000528066. eCollection 2023 Jan. Kidney Dis (Basel). 2022. PMID: 36756081 Free PMC article. Review.
-
The renoprotective effect of esaxerenone independent of blood pressure lowering: a post hoc mediation analysis of the ESAX-DN trial.Hypertens Res. 2023 Feb;46(2):437-444. doi: 10.1038/s41440-022-01008-w. Epub 2022 Sep 13. Hypertens Res. 2023. PMID: 36100672 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

