Efficacy and safety of esaxerenone (CS-3150) in Japanese patients with type 2 diabetes and macroalbuminuria: a multicenter, single-arm, open-label phase III study
- PMID: 34110524
- PMCID: PMC8421271
- DOI: 10.1007/s10157-021-02075-y
Efficacy and safety of esaxerenone (CS-3150) in Japanese patients with type 2 diabetes and macroalbuminuria: a multicenter, single-arm, open-label phase III study
Abstract
Background: Esaxerenone has potential renoprotective effects and reduces the urinary albumin-to-creatinine ratio (UACR) in patients with diabetic kidney disease and overt nephropathy. We investigated the efficacy and safety of esaxerenone in Japanese patients with type 2 diabetes (T2D) and macroalbuminuria (UACR ≥ 300 mg/g creatinine).
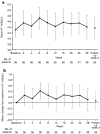
Methods: We conducted a multicenter, single-arm, open-label phase III study in 56 patients with T2D and UACR ≥ 300 mg/g creatinine with estimated glomerular filtration rate (eGFR) ≥ 30 mL/min/1.73 m2 and treated with a renin-angiotensin system inhibitor. Patients received esaxerenone for 28 weeks at 1.25 mg/day initially with titration to 2.5 mg/day based on serum potassium (K+) monitoring. Efficacy was evaluated as the change in UACR from baseline to week 28. Safety endpoints included adverse events (AEs), incidence of serum K+ increase, and change in eGFR from baseline.
Results: UACR decreased by 54.6% (95% CI 46.9%, 61.3%) on average from baseline (544.1 mg/g creatinine) to the end of treatment (246.8 mg/g creatinine); 51.8% of patients showed improvement to early nephropathy. AE incidence was 69.6%. Three patients (5.4%) had serum K+ levels ≥ 6.0 mEq/L or ≥ 5.5 mEq/L on two consecutive occasions. Hyperkalemia in two patients was transient and resolved during the treatment period. One patient discontinued following two consecutive serum K+ values ≥ 5.5 mEq/L. The maximum change from baseline in eGFR was - 8.3 mL/min/1.73 m2 at week 24.
Conclusions: Esaxerenone reduced UACR in Japanese patients with T2D and UACR ≥ 300 mg/g creatinine; more than half experienced a transition from UACR ≥ 300 mg/g creatinine to UACR < 300 mg/g creatinine.
Clinical trial registration: JapicCTI-173696.
Keywords: Esaxerenone; Macroalbuminuria; Phase III; Type 2 diabetes; Urinary albumin-to-creatinine ratio.
© 2021. The Author(s).
Conflict of interest statement
Employment: Yasuyuki Okuda (Daiichi Sankyo Co., Ltd.) and Tomoko Sawanobori (Daiichi Sankyo Co., Ltd); Personal fees: Sadayoshi Ito (Daiichi Sankyo Co., Ltd.), Naoki Kashihara (Daiichi Sankyo Co., Ltd.), Kenichi Shikata (Daiichi Sankyo Co., Ltd.), Masaomi Nangaku (Daiichi Sankyo Co., Ltd.), and Takashi Wada (Daiichi Sankyo Co., Ltd); Grants/research funding received: Sadayoshi Ito (Daiichi Sankyo Co., Ltd.), Naoki Kashihara (Daiichi Sankyo Co., Ltd.), and Masaomi Nangaku (Daiichi Sankyo Co., Ltd).
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

