A Journey with LGMD: From Protein Abnormalities to Patient Impact
- PMID: 34110586
- PMCID: PMC8190568
- DOI: 10.1007/s10930-021-10006-9
A Journey with LGMD: From Protein Abnormalities to Patient Impact
Abstract
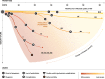
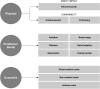
The limb-girdle muscular dystrophies (LGMD) are a collection of genetic diseases united in their phenotypical expression of pelvic and shoulder area weakness and wasting. More than 30 subtypes have been identified, five dominant and 26 recessive. The increase in the characterization of new genotypes in the family of LGMDs further adds to the heterogeneity of the disease. Meanwhile, better understanding of the phenotype led to the reconsideration of the disease definition, which resulted in eight old subtypes to be no longer recognized officially as LGMD and five new diseases to be added to the LGMD family. The unique variabilities of LGMD stem from genetic mutations, which then lead to protein and ultimately muscle dysfunction. Herein, we review the LGMD pathway, starting with the genetic mutations that encode proteins involved in muscle maintenance and repair, and including the genotype-phenotype relationship of the disease, the epidemiology, disease progression, burden of illness, and emerging treatments.
Keywords: Anoctaminopathy—LGMD R12 (2L); Calpainopathy—LGMD R1 (2A); Dysferlinopathy—LGMD R2 (2B); Dystroglycanopathies—LGMD R9 (2I); Limb-girdle muscular dystrophy (LGMD); Sarcoglycanopathies—LGMD R3-6 (2C-2F).
© 2021. The Author(s).
Figures


References
-
- Kroneman M, de Visser M (2019) Patient information leaflet on new names for LGMD. European Reference Network—EURO-NMD. Retrieved February 28, 2021, from https://ern-euro-nmd.eu/ern/wp-content/uploads/2019/10/New_names_for_lim...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

