Distinct antigen uptake receptors route to the same storage compartments for cross-presentation in dendritic cells
- PMID: 34110622
- PMCID: PMC8517591
- DOI: 10.1111/imm.13382
Distinct antigen uptake receptors route to the same storage compartments for cross-presentation in dendritic cells
Abstract
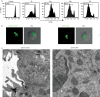
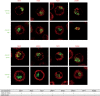
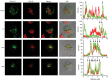
An exclusive feature of dendritic cells (DCs) is their capacity to present exogenous antigens by MHC class I molecules, called cross-presentation. Here, we show that protein antigen can be conserved in mature murine DCs for several days in a lysosome-like storage compartment, distinct from MHC class II and early endosomal compartments, as an internal source for the supply of MHC class I ligands. Using two different uptake routes via Fcγ receptors and C-type lectin receptors, we could show that antigens were routed towards the same endolysosomal compartments after 48 h. The antigen-containing compartments lacked co-expression of molecules involved in MHC class I processing and presentation including TAP and proteasome subunits as shown by single-cell imaging flow cytometry. Moreover, we observed the absence of cathepsin S but selective co-localization of active cathepsin X with protein antigen in the storage compartments. This indicates cathepsin S-independent antigen degradation and a novel but yet undefined role for cathepsin X in antigen processing and cross-presentation by DCs. In summary, our data suggest that these antigen-containing compartments in DCs can conserve protein antigens from different uptake routes and contribute to long-lasting antigen cross-presentation.
Keywords: C-type lectin receptors; Fc receptors; antigen cross-presentation; cathepsin; dendritic cells.
© 2021 The Authors. Immunology published by John Wiley & Sons Ltd.
Conflict of interest statement
There are no competing interests.
Figures



Comment in
-
A specialist antigen storage compartment in dendritic cells to sustain cross-presentation.Immunology. 2021 Nov;164(3):399-400. doi: 10.1111/imm.13422. Immunology. 2021. PMID: 34651319 Free PMC article. No abstract available.
Similar articles
-
Redirecting soluble antigen for MHC class I cross-presentation during phagocytosis.Eur J Immunol. 2015 Feb;45(2):383-95. doi: 10.1002/eji.201445156. Epub 2014 Dec 8. Eur J Immunol. 2015. PMID: 25378230 Free PMC article.
-
Antigen delivery to early endosomes eliminates the superiority of human blood BDCA3+ dendritic cells at cross presentation.J Exp Med. 2013 May 6;210(5):1049-63. doi: 10.1084/jem.20121251. Epub 2013 Apr 8. J Exp Med. 2013. PMID: 23569326 Free PMC article.
-
Antigen storage compartments in mature dendritic cells facilitate prolonged cytotoxic T lymphocyte cross-priming capacity.Proc Natl Acad Sci U S A. 2009 Apr 21;106(16):6730-5. doi: 10.1073/pnas.0900969106. Epub 2009 Apr 3. Proc Natl Acad Sci U S A. 2009. PMID: 19346487 Free PMC article.
-
The ins-and-outs of endosomal antigens for cross-presentation.Curr Opin Immunol. 2014 Feb;26:63-8. doi: 10.1016/j.coi.2013.11.001. Epub 2013 Nov 30. Curr Opin Immunol. 2014. PMID: 24556402 Review.
-
Endocytic Recycling of MHC Class I Molecules in Non-professional Antigen Presenting and Dendritic Cells.Front Immunol. 2019 Jan 7;9:3098. doi: 10.3389/fimmu.2018.03098. eCollection 2018. Front Immunol. 2019. PMID: 30666258 Free PMC article. Review.
Cited by
-
Straight to the point: targeted mRNA-delivery to immune cells for improved vaccine design.Front Immunol. 2023 Nov 27;14:1294929. doi: 10.3389/fimmu.2023.1294929. eCollection 2023. Front Immunol. 2023. PMID: 38090568 Free PMC article. Review.
-
Advances in vaccine development for Chlamydia trachomatis.Pathog Dis. 2024 Feb 7;82:ftae017. doi: 10.1093/femspd/ftae017. Pathog Dis. 2024. PMID: 39043447 Free PMC article. Review.
-
A specialist antigen storage compartment in dendritic cells to sustain cross-presentation.Immunology. 2021 Nov;164(3):399-400. doi: 10.1111/imm.13422. Immunology. 2021. PMID: 34651319 Free PMC article. No abstract available.
-
Major histocompatibility complex class I assembly within endolysosomal pathways.Curr Opin Immunol. 2023 Oct;84:102356. doi: 10.1016/j.coi.2023.102356. Epub 2023 Jun 26. Curr Opin Immunol. 2023. PMID: 37379719 Free PMC article. Review.
-
Tug of war: Understanding the dynamic interplay of tumor biomechanical environment on dendritic cell function.Mechanobiol Med. 2024 Apr 27;2(3):100068. doi: 10.1016/j.mbm.2024.100068. eCollection 2024 Sep. Mechanobiol Med. 2024. PMID: 40395498 Free PMC article. Review.
References
-
- Shen L, Sigal LJ, Boes M, Rock KL. Important role of cathepsin S in generating peptides for TAP‐independent MHC class I crosspresentation in vivo. Immunity. 2004;21:155–65. - PubMed
-
- Kovacsovics‐Bankowski M, Rock KL. A phagosome‐to‐cytosol pathway for exogenous antigens presented on MHC class I molecules. Science (80). 1995;267:243–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

