PAI-1 induction during kidney injury promotes fibrotic epithelial dysfunction via deregulation of klotho, p53, and TGF-β1-receptor signaling
- PMID: 34110636
- PMCID: PMC8594377
- DOI: 10.1096/fj.202002652RR
PAI-1 induction during kidney injury promotes fibrotic epithelial dysfunction via deregulation of klotho, p53, and TGF-β1-receptor signaling
Abstract
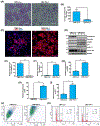
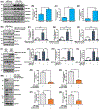
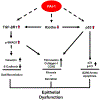
Renal fibrosis leads to chronic kidney disease, which affects over 15% of the U.S. population. PAI-1 is highly upregulated in the tubulointerstitial compartment in several common nephropathies and PAI-1 global ablation affords protection from fibrogenesis in mice. The precise contribution of renal tubular PAI-1 induction to disease progression, however, is unknown and surprisingly, appears to be independent of uPA inhibition. Human renal epithelial (HK-2) cells engineered to stably overexpress PAI-1 underwent dedifferentiation (E-cadherin loss, gain of vimentin), G2/M growth arrest (increased p-Histone3, p21), and robust induction of fibronectin, collagen-1, and CCN2. These cells are also susceptible to apoptosis (elevated cleaved caspase-3, annexin-V positivity) compared to vector controls, demonstrating a previously unknown role for PAI-1 in tubular dysfunction. Persistent PAI-1 expression results in a loss of klotho expression, p53 upregulation, and increases in TGF-βRI/II levels and SMAD3 phosphorylation. Ectopic restoration of klotho in PAI-1-transductants attenuated fibrogenesis and reversed the proliferative defects, implicating PAI-1 in klotho loss in renal disease. Genetic suppression of p53 reversed the PA1-1-driven maladaptive repair, moreover, confirming a pathogenic role for p53 upregulation in this context and uncovering a novel role for PAI-1 in promoting renal p53 signaling. TGF-βRI inhibition also attenuated PAI-1-initiated epithelial dysfunction, independent of TGF-β1 ligand synthesis. Thus, PAI-1 promotes tubular dysfunction via klotho reduction, p53 upregulation, and activation of the TGF-βRI-SMAD3 axis. Since klotho is an upstream regulator of both PAI-1-mediated p53 induction and SMAD3 signaling, targeting tubular PAI-1 expression may provide a novel, multi-level approach to the therapy of CKD.
Keywords: PAI-1; TGF-β1; cell cycle arrest; chronic kidney disease; epithelial dysfunction; klotho; obstructive nephropathy; p53; renal fibrosis.
© 2021 Federation of American Societies for Experimental Biology.
Conflict of interest statement
CONFLICT OF INTEREST
All authors declared no conflict of interest and agreed on the manuscript prior to submission.
Figures






Similar articles
-
Rac-GTPase promotes fibrotic TGF-β1 signaling and chronic kidney disease via EGFR, p53, and Hippo/YAP/TAZ pathways.FASEB J. 2019 Sep;33(9):9797-9810. doi: 10.1096/fj.201802489RR. Epub 2019 May 16. FASEB J. 2019. PMID: 31095421 Free PMC article.
-
Redox control of p53 in the transcriptional regulation of TGF-β1 target genes through SMAD cooperativity.Cell Signal. 2014 Jul;26(7):1427-36. doi: 10.1016/j.cellsig.2014.02.017. Epub 2014 Mar 5. Cell Signal. 2014. PMID: 24613410 Free PMC article.
-
Tumor suppressor ataxia telangiectasia mutated functions downstream of TGF-β1 in orchestrating profibrotic responses.FASEB J. 2015 Apr;29(4):1258-68. doi: 10.1096/fj.14-262527. Epub 2014 Dec 5. FASEB J. 2015. PMID: 25480384 Free PMC article.
-
TGF-β1-p53 cooperativity regulates a profibrotic genomic program in the kidney: molecular mechanisms and clinical implications.FASEB J. 2019 Oct;33(10):10596-10606. doi: 10.1096/fj.201900943R. Epub 2019 Jul 6. FASEB J. 2019. PMID: 31284746 Free PMC article. Review.
-
The TGF-β1/p53/PAI-1 Signaling Axis in Vascular Senescence: Role of Caveolin-1.Biomolecules. 2019 Aug 3;9(8):341. doi: 10.3390/biom9080341. Biomolecules. 2019. PMID: 31382626 Free PMC article. Review.
Cited by
-
Kidney-specific methylation patterns correlate with kidney function and are lost upon kidney disease progression.Clin Epigenetics. 2024 Feb 12;16(1):27. doi: 10.1186/s13148-024-01642-w. Clin Epigenetics. 2024. PMID: 38347603 Free PMC article.
-
Association of thrombomodulin with the severity of chronic kidney disease: a cross-sectional study.BMC Nephrol. 2025 May 30;26(1):268. doi: 10.1186/s12882-025-04200-5. BMC Nephrol. 2025. PMID: 40448261 Free PMC article.
-
Role of the TGF‑β/Smad signaling pathway in the transition from acute kidney injury to chronic kidney disease (Review).Int J Mol Med. 2025 Oct;56(4):162. doi: 10.3892/ijmm.2025.5603. Epub 2025 Aug 1. Int J Mol Med. 2025. PMID: 40747672 Free PMC article. Review.
-
Isolation and characterization mesenchymal stem cells from red panda (Ailurus fulgens styani) endometrium.Conserv Physiol. 2022 Feb 21;10(1):coac004. doi: 10.1093/conphys/coac004. eCollection 2022 Jan 1. Conserv Physiol. 2022. PMID: 35211318 Free PMC article.
-
Protein-Bound Uremic Toxins in Senescence and Kidney Fibrosis.Biomedicines. 2023 Aug 28;11(9):2408. doi: 10.3390/biomedicines11092408. Biomedicines. 2023. PMID: 37760849 Free PMC article. Review.
References
-
- Centers for disease control and prevention. https://www.cdc.gov/kidneydisease/basics.html
-
- Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. - PubMed
-
- Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommuni-cable diseases. Kidney Int. 2011;80:1258–1270. - PubMed
-
- Perico N, Remuzzi G. Chronic kidney disease: a research and public health priority. Nephrol Dial Transplant. 2012;27(Suppl 3):19–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

