Decreases in COVID-19 Cases, Emergency Department Visits, Hospital Admissions, and Deaths Among Older Adults Following the Introduction of COVID-19 Vaccine - United States, September 6, 2020-May 1, 2021
- PMID: 34111059
- PMCID: PMC8191865
- DOI: 10.15585/mmwr.mm7023e2
Decreases in COVID-19 Cases, Emergency Department Visits, Hospital Admissions, and Deaths Among Older Adults Following the Introduction of COVID-19 Vaccine - United States, September 6, 2020-May 1, 2021
Abstract
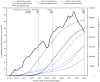
Throughout the COVID-19 pandemic, older U.S. adults have been at increased risk for severe COVID-19-associated illness and death (1). On December 14, 2020, the United States began a nationwide vaccination campaign after the Food and Drug Administration's Emergency Use Authorization of Pfizer-BioNTech COVID-19 vaccine. The Advisory Committee on Immunization Practices (ACIP) recommended prioritizing health care personnel and residents of long-term care facilities, followed by essential workers and persons at risk for severe illness, including adults aged ≥65 years, in the early phases of the vaccination program (2). By May 1, 2021, 82%, 63%, and 42% of persons aged ≥65, 50-64, and 18-49 years, respectively, had received ≥1 COVID-19 vaccine dose. CDC calculated the rates of COVID-19 cases, emergency department (ED) visits, hospital admissions, and deaths by age group during November 29-December 12, 2020 (prevaccine) and April 18-May 1, 2021. The rate ratios comparing the oldest age groups (≥70 years for hospital admissions; ≥65 years for other measures) with adults aged 18-49 years were 40%, 59%, 65%, and 66% lower, respectively, in the latter period. These differential declines are likely due, in part, to higher COVID-19 vaccination coverage among older adults, highlighting the potential benefits of rapidly increasing vaccination coverage.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures

References
-
- Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci 1986;1:54–75. 10.1214/ss/1177013815 - DOI
-
- Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021;397:1819–29. 10.1016/S0140-6736(21)00947-8 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

