Translation stress and collided ribosomes are co-activators of cGAS
- PMID: 34111399
- PMCID: PMC8260207
- DOI: 10.1016/j.molcel.2021.05.018
Translation stress and collided ribosomes are co-activators of cGAS
Abstract
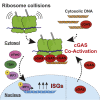
The cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway senses cytosolic DNA and induces interferon-stimulated genes (ISGs) to activate the innate immune system. Here, we report the unexpected discovery that cGAS also senses dysfunctional protein production. Purified ribosomes interact directly with cGAS and stimulate its DNA-dependent activity in vitro. Disruption of the ribosome-associated protein quality control (RQC) pathway, which detects and resolves ribosome collision during translation, results in cGAS-dependent ISG expression and causes re-localization of cGAS from the nucleus to the cytosol. Indeed, cGAS preferentially binds collided ribosomes in vitro, and orthogonal perturbations that result in elevated levels of collided ribosomes and RQC activation cause sub-cellular re-localization of cGAS and ribosome binding in vivo as well. Thus, translation stress potently increases DNA-dependent cGAS activation. These findings have implications for the inflammatory response to viral infection and tumorigenesis, both of which substantially reprogram cellular protein synthesis.
Keywords: ASCC3; IRF3; STING; ZNF598; cGAS; innate immunity; interferon signalling; mRNA translation; ribosome collision; ribosome-associated protein quality control.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures






References
-
- Ablasser A., Chen Z.J. cGAS in action: Expanding roles in immunity and inflammation. Science. 2019;363:eaat8657. - PubMed
-
- Ablasser A., Hertrich C., Waßermann R., Hornung V. Nucleic acid driven sterile inflammation. Clin. Immunol. 2013;147:207–215. - PubMed
-
- Andreeva L., Hiller B., Kostrewa D., Lässig C., de Oliveira Mann C.C., Jan Drexler D., Maiser A., Gaidt M., Leonhardt H., Hornung V., Hopfner K.P. cGAS senses long and HMGB/TFAM-bound U-turn DNA by forming protein-DNA ladders. Nature. 2017;549:394–398. - PubMed
-
- Arquint C., Nigg E.A. STIL microcephaly mutations interfere with APC/C-mediated degradation and cause centriole amplification. Curr. Biol. 2014;24:351–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

