An ammonium transporter is a non-canonical olfactory receptor for ammonia
- PMID: 34111404
- PMCID: PMC8355169
- DOI: 10.1016/j.cub.2021.05.025
An ammonium transporter is a non-canonical olfactory receptor for ammonia
Abstract
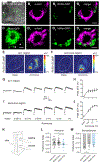
Numerous hematophagous insects are attracted to ammonia, a volatile released in human sweat and breath.1-3 Low levels of ammonia also attract non-biting insects such as the genetic model organism Drosophila melanogaster and several species of agricultural pests.4,5 Two families of ligand-gated ion channels function as olfactory receptors in insects,6-10 and studies have linked ammonia sensitivity to a particular olfactory receptor in Drosophila.5,11,12 Given the widespread importance of ammonia to insect behavior, it is surprising that the genomes of most insects lack an ortholog of this gene.6 Here, we show that canonical olfactory receptors are not necessary for responses to ammonia in Drosophila. Instead, we demonstrate that a member of the ancient electrogenic ammonium transporter family, Amt, is likely a new type of olfactory receptor. We report two hitherto unidentified olfactory neuron populations that mediate neuronal and behavioral responses to ammonia in Drosophila. Their endogenous ammonia responses are lost in Amt mutant flies, and ectopic expression of either Drosophila or Anopheles Amt confers ammonia sensitivity. These results suggest that Amt is the first transporter known to function as an olfactory receptor in animals and that its function may be conserved across insect species.
Keywords: Amt; Drosophila; ammonia; mosquito; olfactory behavior; olfactory receptor; olfactory receptor neurons; sacculus; transporter.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Mumcuoglu Y, Galun R, and Ikan R (1986). The aggregation response of human body louse (Pediculus humanus)(Insecta: Anoplura) to its excretory products. Int. J.Trop. Insect Sci. 7, 629–632.
-
- Takken W, and Knols BGJ (2010). Olfaction in Vector-Host Interactions, Volume 2, (Wageningen, The Netherlands: Wageningen Academic Publishers; ).
-
- Andrade AJ, Andrade MR, Dias ES, Pinto MC, and Eiras AE (2008). Are light traps baited with kairomones effective in the capture of Lutzomyia longipalpis and Lutzomyia intermedia? An evaluation of synthetic human odor as an attractant for phlebotomine sand flies (Diptera: Psychodidae: Phlebotominae). Mem. Inst. Oswaldo Cruz 103, 337–343. - PubMed
-
- Epsky ND, Kendra PE, and Schnell EQ (2014). History and Development of Food-Based Attractants. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies, Shelly T, Epsky N, Jang EB, Reyes-Flores J and Vargas R, eds. (Dordrecht, Netherlands: Springer; ), pp. 75–118.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

