Arterolane-piperaquine-mefloquine versus arterolane-piperaquine and artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children: a single-centre, open-label, randomised, non-inferiority trial
- PMID: 34111412
- PMCID: PMC8461080
- DOI: 10.1016/S1473-3099(20)30929-4
Arterolane-piperaquine-mefloquine versus arterolane-piperaquine and artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children: a single-centre, open-label, randomised, non-inferiority trial
Abstract
Background: Triple antimalarial combination therapies combine potent and rapidly cleared artemisinins or related synthetic ozonides, such as arterolane, with two, more slowly eliminated partner drugs to reduce the risk of resistance. We aimed to assess the safety, tolerability, and efficacy of arterolane-piperaquine-mefloquine versus arterolane-piperaquine and artemether-lumefantrine for the treatment of uncomplicated falciparum malaria in Kenyan children.
Methods: In this single-centre, open-label, randomised, non-inferiority trial done in Kilifi County Hospital, Kilifi, coastal Kenya, children with uncomplicated Plasmodium falciparum malaria were recruited. Eligible patients were aged 2-12 years and had an asexual parasitaemia of 5000-250 000 parasites per μL. The exclusion criteria included the presence of an acute illness other than malaria, the inability to tolerate oral medications, treatment with an artemisinin derivative in the previous 7 days, a known hypersensitivity or contraindication to any of the study drugs, and a QT interval corrected for heart rate (QTc interval) longer than 450 ms. Patients were randomly assigned (1:1:1), by use of blocks of six, nine, and 12, and opaque, sealed, and sequentially numbered envelopes, to receive either arterolane-piperaquine, arterolane-piperaquine-mefloquine, or artemether-lumefantrine. Laboratory staff, but not the patients, the patients' parents or caregivers, clinical or medical officers, nurses, or trial statistician, were masked to the intervention groups. For 3 days, oral artemether-lumefantrine was administered twice daily (target dose 5-24 mg/kg of bodyweight of artemether and 29-144 mg/kg of bodyweight of lumefantrine), and oral arterolane-piperaquine (arterolane dose 4 mg/kg of bodyweight; piperaquine dose 20 mg/kg of bodyweight) and oral arterolane-piperaquine-mefloquine (mefloquine dose 8 mg/kg of bodyweight) were administered once daily. All patients received 0·25 mg/kg of bodyweight of oral primaquine at hour 24. All patients were admitted to Kilifi County Hospital for at least 3 consecutive days and followed up at day 7 and, thereafter, weekly for up to 42 days. The primary endpoint was 42-day PCR-corrected efficacy, defined as the absence of treatment failure in the first 42 days post-treatment, of arterolane-piperaquine-mefloquine versus artemether-lumefantrine, and, along with safety, was analysed in the intention-to-treat population, which comprised all patients who received at least one dose of a study drug. The 42-day PCR-corrected efficacy of arterolane-piperaquine-mefloquine versus arterolane-piperaquine was an important secondary endpoint and was also analysed in the intention-to-treat population. The non-inferiority margin for the risk difference between treatments was -7%. The study is registered in ClinicalTrials.gov, NCT03452475, and is completed.
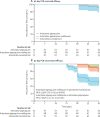
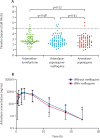
Findings: Between March 7, 2018, and May 2, 2019, 533 children with P falciparum were screened, of whom 217 were randomly assigned to receive either arterolane-piperaquine (n=73), arterolane-piperaquine-mefloquine (n=72), or artemether-lumefantrine (n=72) and comprised the intention-to-treat population. The 42-day PCR-corrected efficacy after treatment with arterolane-piperaquine-mefloquine (100%, 95% CI 95-100; 72/72) was non-inferior to that after treatment with artemether-lumefantrine (96%, 95% CI 88-99; 69/72; risk difference 4%, 95% CI 0-9; p=0·25). The 42-day PCR-corrected efficacy of arterolane-piperaquine-mefloquine was non-inferior to that of arterolane-piperaquine (100%, 95% CI 95-100; 73/73; risk difference 0%). Vomiting rates in the first hour post-drug administration were significantly higher in patients treated with arterolane-piperaquine (5%, 95% CI 2-9; ten of 203 drug administrations; p=0·0013) or arterolane-piperaquine-mefloquine (5%, 3-9; 11 of 209 drug administrations; p=0·0006) than in patients treated with artemether-lumefantrine (1%, 0-2; three of 415 drug administrations). Upper respiratory tract complaints (n=26 for artemether-lumefantrine; n=19 for arterolane-piperaquine-mefloquine; n=23 for arterolane-piperaquine), headache (n=13; n=4; n=5), and abdominal pain (n=7; n=5; n=5) were the most frequently reported adverse events. There were no deaths.
Interpretation: This study shows that arterolane-piperaquine-mefloquine is an efficacious and safe treatment for uncomplicated falciparum malaria in children and could potentially be used to prevent or delay the emergence of antimalarial resistance.
Funding: UK Department for International Development, The Wellcome Trust, The Bill & Melinda Gates Foundation, Sun Pharmaceutical Industries.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures

Comment in
-
Is triple artemisinin-based combination therapy necessary for uncomplicated malaria?Lancet Infect Dis. 2022 May;22(5):585-586. doi: 10.1016/S1473-3099(22)00208-0. Lancet Infect Dis. 2022. PMID: 35460649 No abstract available.
-
Is triple artemisinin-based combination therapy necessary for uncomplicated malaria?Lancet Infect Dis. 2022 Jun;22(6):765-766. doi: 10.1016/S1473-3099(22)00283-3. Lancet Infect Dis. 2022. PMID: 35643100 Free PMC article. No abstract available.
References
-
- Wootton JC, Feng X, Ferdig MT. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature. 2002;418:320–323. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

