Chemokines and eicosanoids fuel the hyperinflammation within the lungs of patients with severe COVID-19
- PMID: 34111453
- PMCID: PMC8180473
- DOI: 10.1016/j.jaci.2021.05.032
Chemokines and eicosanoids fuel the hyperinflammation within the lungs of patients with severe COVID-19
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can lead to a variety of clinical outcomes, ranging from the absence of symptoms to severe acute respiratory disease and ultimately death. A feature of patients with severe coronavirus disease 2019 (COVID-19) is the abundance of inflammatory cytokines in the blood. Elevated levels of cytokines are predictive of infection severity and clinical outcome. In contrast, studies aimed at defining the driving forces behind the inflammation in lungs of subjects with severe COVID-19 remain scarce.
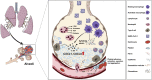
Objective: Our aim was to analyze and compare the plasma and bronchoalveolar lavage (BAL) fluids of patients with severe COVID-19 (n = 45) for the presence of cytokines and lipid mediators of inflammation (LMIs).
Methods: Cytokines were measured by using Luminex multiplex assay, and LMIs were measured by using liquid chromatography-tandem mass spectrometry.
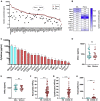
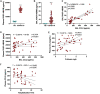
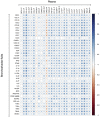
Results: We revealed high concentrations of numerous cytokines, chemokines, and LMIs in the BAL fluid of patients with severe COVID-19. Of the 13 most abundant mediators in BAL fluid, 11 were chemokines, with CXCL1 and CXCL8 being 200 times more abundant than IL-6 and TNF-α. Eicosanoid levels were also elevated in the lungs of subjects with severe COVID-19. Consistent with the presence chemotactic molecules, BAL fluid samples were enriched for neutrophils, lymphocytes, and eosinophils. Inflammatory cytokines and LMIs in plasma showed limited correlations with those present in BAL fluid, arguing that circulating inflammatory molecules may not be a reliable proxy of the inflammation occurring in the lungs of patients with severe COVID-19.
Conclusions: Our findings indicate that hyperinflammation of the lungs of patients with severe COVID-19 is fueled by excessive production of chemokines and eicosanoids. Therapeutic strategies to dampen inflammation in patients with COVID-19 should be tailored accordingly.
Keywords: ARDS; COVID-19; SARS-CoV-2; chemokines; eicosanoids; inflammatory cytokines; lipid mediators of inflammation.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

