Phosphorylation of TRIP13 at Y56 induces radiation resistance but sensitizes head and neck cancer to cetuximab
- PMID: 34111559
- PMCID: PMC8753291
- DOI: 10.1016/j.ymthe.2021.06.009
Phosphorylation of TRIP13 at Y56 induces radiation resistance but sensitizes head and neck cancer to cetuximab
Abstract
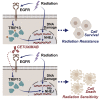
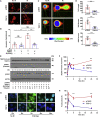
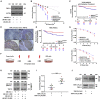
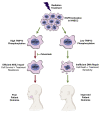
Radiation therapy, a mainstay of treatment for head and neck cancer, is not always curative due to the development of treatment resistance; additionally, multi-institutional trials have questioned the efficacy of concurrent radiation with cetuximab, the epidermal growth factor receptor (EGFR) inhibitor. We unraveled a mechanism for radiation resistance; that is, radiation induces EGFR, which phosphorylates TRIP13 (thyroid hormone receptor interactor 13) on tyrosine 56. Phosphorylated (phospho-)TRIP13 promotes non-homologous end joining (NHEJ) repair to induce radiation resistance. NHEJ is the main repair pathway for radiation-induced DNA damage. Tumors expressing high TRIP13 do not respond to radiation but are sensitive to cetuximab or cetuximab combined with radiation. Suppression of phosphorylation of TRIP13 at Y56 abrogates these effects. These findings show that EGFR-mediated phosphorylation of TRIP13 at Y56 is a vital mechanism of radiation resistance. Notably, TRIP13-pY56 could be used to predict the response to radiation or cetuximab and could be explored as an actionable target.
Keywords: DSB repair; EGFR; NHEJ; TRIP13; cetuximab; radiation resistance; squamous cell carcinoma.
Copyright © 2021 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Leemans C.R., Snijders P.J.F., Brakenhoff R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer. 2018;18:269–282. - PubMed
-
- Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Piñeros M., Znaor A., Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer. 2019;144:1941–1953. - PubMed
-
- Perch S.J., Machtay M., Markiewicz D.A., Kligerman M.M. Decreased acute toxicity by using midline mucosa-sparing blocks during radiation therapy for carcinoma of the oral cavity, oropharynx, and nasopharynx. Radiology. 1995;197:863–866. - PubMed
-
- Ang K.K., Berkey B.A., Tu X., Zhang H.Z., Katz R., Hammond E.H., Fu K.K., Milas L. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

