Quantitative SARS-CoV-2 anti-spike responses to Pfizer-BioNTech and Oxford-AstraZeneca vaccines by previous infection status
- PMID: 34111577
- PMCID: PMC8180449
- DOI: 10.1016/j.cmi.2021.05.041
Quantitative SARS-CoV-2 anti-spike responses to Pfizer-BioNTech and Oxford-AstraZeneca vaccines by previous infection status
Abstract
Objectives: We investigated determinants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) anti-spike IgG responses in healthcare workers (HCWs) following one or two doses of Pfizer-BioNTech or Oxford-AstraZeneca vaccines.
Methods: HCWs participating in regular SARS-CoV-2 PCR and antibody testing were invited for serological testing prior to first and second vaccination, and 4 weeks post-vaccination if receiving a 12-week dosing interval. Quantitative post-vaccination anti-spike antibody responses were measured using the Abbott SARS-CoV-2 IgG II Quant assay (detection threshold: ≥50 AU/mL). We used multivariable logistic regression to identify predictors of seropositivity and generalized additive models to track antibody responses over time.
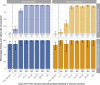
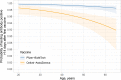
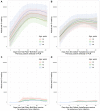
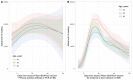
Results: 3570/3610 HCWs (98.9%) were seropositive >14 days post first vaccination and prior to second vaccination: 2706/2720 (99.5%) were seropositive after the Pfizer-BioNTech and 864/890 (97.1%) following the Oxford-AstraZeneca vaccines. Previously infected and younger HCWs were more likely to test seropositive post first vaccination, with no evidence of differences by sex or ethnicity. All 470 HCWs tested >14 days after the second vaccination were seropositive. Quantitative antibody responses were higher after previous infection: median (IQR) >21 days post first Pfizer-BioNTech 14 604 (7644-22 291) AU/mL versus 1028 (564-1985) AU/mL without prior infection (p < 0.001). Oxford-AstraZeneca vaccine recipients had lower readings post first dose than Pfizer-BioNTech recipients, with and without previous infection, 10 095 (5354-17 096) and 435 (203-962) AU/mL respectively (both p < 0.001 versus Pfizer-BioNTech). Antibody responses >21 days post second Pfizer vaccination in those not previously infected, 10 058 (6408-15 582) AU/mL, were similar to those after prior infection followed by one vaccine dose.
Conclusions: SARS-CoV-2 vaccination leads to detectable anti-spike antibodies in nearly all adult HCWs. Whether differences in response impact vaccine efficacy needs further study.
Keywords: Antibody; Quantitative anti-spike antibody; SARS-CoV-2; Serology; Vaccine.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Medicines and Healthcare products Regulatory Agency . 2020. MHRA guidance on coronavirus (COVID-19)https://www.gov.uk/government/collections/mhra-guidance-on-coronavirus-c...
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

