Impaired Binding to Junctophilin-2 and Nanostructural Alteration in CPVT Mutation
- PMID: 34111951
- PMCID: PMC8320243
- DOI: 10.1161/CIRCRESAHA.121.319094
Impaired Binding to Junctophilin-2 and Nanostructural Alteration in CPVT Mutation
Abstract
[Figure: see text].
Keywords: action potential; calcium; junctophilin; mutation; ryanodine receptor; ventricular tachycardia.
Conflict of interest statement
DISCLOSURES
No conflict of interest
Figures
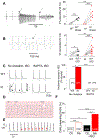


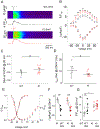


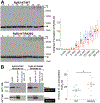
Comment in
-
RyR2 Gain-of-Function and Not So Sudden Cardiac Death.Circ Res. 2021 Jul 23;129(3):417-419. doi: 10.1161/CIRCRESAHA.121.319651. Epub 2021 Jul 22. Circ Res. 2021. PMID: 34292783 Free PMC article. No abstract available.
References
-
- Mehra R Global public health problem of sudden cardiac death. Journal of electrocardiology. 2007;40:S118–122 - PubMed
-
- Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91:1512–1519 - PubMed
-
- Peng W, Shen H, Wu J, Guo W, Pan X, Wang R, Chen SR, Yan N. Structural basis for the gating mechanism of the type 2 ryanodine receptor ryr2. Science. 2016;354 - PubMed
-
- George CH JH, Thomas NL, Fry DL, Lai FA. Ryanodine receptors and ventricular arrhythmias: Emerging trends in mutations, mechanisms and therapies. J Mol and Cell Cardiol. 2007;42:34–50 - PubMed
-
- Yano M, Yamamoto T, Ikeda Y, Matsuzaki M. Mechanisms of disease: Ryanodine receptor defects in heart failure and fatal arrhythmia. Nat Clin Pract Cardiovasc Med. 2006;3:43–52 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

