Imaging of inflammatory disease of the pancreas
- PMID: 34111970
- PMCID: PMC8248196
- DOI: 10.1259/bjr.20201214
Imaging of inflammatory disease of the pancreas
Abstract
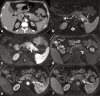
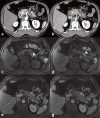
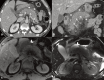
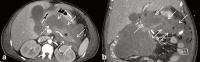
Increasingly acute and chronic pancreatitis (AP and CP) are considered a continuum of a single entity. Nonetheless, if, after flare-up, the pancreas shows no residual inflammation, it is classified as AP. CP is characterised by a long cycle of worsening and waning glandular inflammation without the pancreas ever returning to its baseline structure or function. According to the International Consensus Guidelines on Early Chronic Pancreatitis, pancreatic inflammation must last at least 6 months before it can be labelled CP. The distinction is important because, unlike AP, CP can destroy endocrine and exocrine pancreatic function, emphasising the importance of early diagnosis. As typical AP can be diagnosed by clinical symptoms plus laboratory tests, imaging is usually reserved for those with recurrent, complicated or CP. Imaging typically starts with ultrasound and more frequently with contrast-enhanced computed tomography (CECT). MRI and/or MR cholangiopancreatography can be used as a problem-solving tool to confirm indirect signs of pancreatic mass, differentiate between solid and cystic lesions, and to exclude pancreatic duct anomalies, as may occur with recurrent AP, or to visualise early signs of CP. MR cholangiopancreatography has replaced diagnostic endoscopic retrograde cholangiopancreatography (ERCP). However, ERCP, and/or endoscopic ultrasound (EUS) remain necessary for transpapillary biliary or pancreatic duct stenting and transgastric cystic fluid drainage or pancreatic tissue sampling, respectively. Finally, positron emission tomography-MRI or positron emission tomography-CT are usually reserved for complicated cases and/or to search for extra pancreatic systemic manifestations. In this article, we discuss a broad spectrum of inflammatory pancreatic disorders and the utility of various modalities in diagnosing acute and chronic pancreatitis.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

