Topical estrogen, testosterone, and vaginal dilator in the prevention of vaginal stenosis after radiotherapy in women with cervical cancer: a randomized clinical trial
- PMID: 34112100
- PMCID: PMC8191143
- DOI: 10.1186/s12885-021-08274-w
Topical estrogen, testosterone, and vaginal dilator in the prevention of vaginal stenosis after radiotherapy in women with cervical cancer: a randomized clinical trial
Erratum in
-
Correction to: Topical estrogen, testosterone, and vaginal dilator in the prevention of vaginal stenosis after radiotherapy in women with cervical cancer: a randomized clinical trial.BMC Cancer. 2021 Jul 15;21(1):811. doi: 10.1186/s12885-021-08543-8. BMC Cancer. 2021. PMID: 34266416 Free PMC article. No abstract available.
Abstract
Background: We aimed to evaluate the effects of different therapeutic options to prevent the evolution of vaginal stenosis after pelvic radiotherapy in women with cervical cancer.
Methods: open-label randomized clinical trial of 195 women, stage I-IIIB, aged 18-75 years, using topical estrogen (66), topical testosterone (34), water-based intimate lubricant gel (66), and vaginal dilators (29) to assess the incidence and severity of vaginal stenosis after radiotherapy at UNICAMP-Brazil, from January/2013 to May/2018. The main outcome measure was vaginal stenosis assessed using the Common Terminology Criteria for Adverse Events (CTCAE) scale and percental changes in vaginal volume. The women were evaluated at four different times: shortly after the end of radiotherapy, and four, eight, and 12 months after the beginning of the intervention. Statistical analysis was carried out using Symmetry test, Kruskal-Wallis test and multiple regression.
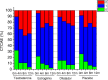
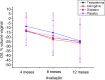
Results: the mean age of women was 46.78 (±13.01) years, 61,03% were premenopausal and 73,84% had stage IIB-IIIB tumors. The mean reduction in vaginal volume in the total group was 25.47%, with similar worsening in the four treatment groups with no statistical difference throughout the intervention period. There was worsening of vaginal stenosis evaluated by CTCAE scale after 1 year in all groups (p < 0.01), except for the users of vaginal dilator (p = 0.37).
Conclusions: there was a reduction in vaginal volume in all treatment groups analyzed, with no significant difference between them. However, women who used vaginal dilators had a lower frequency and severity of vaginal stenosis assessed by the CTCAE scale after one year of treatment.
Trial registration: Brazilian Registry of Clinical Trials, RBR-23w5fv . Registered 10 January 2017 - Retrospectively registered.
Keywords: Adverse effects; Brachytherapy; Radiotherapy; Uterine cervical neoplasm.
Conflict of interest statement
The authors have no conflicts of interest.
Figures
References
-
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. GLOBOCAN 2018, Cancer today. Lyon: IARC; 2018.
-
- World Health Organization. International Agency for Research on Cancer: GLOBOCAN; 2018. Available at: https://gco.iarc.fr/today/online-analysis-table. Accessed 29 Dec 2019
-
- Instituto Nacional de Câncer José Alencar Gomes da Silva/ Ministério da Saúde (Brasil) Estimate/2018 – Cancer Incidence in Brazil. 2018.
-
- Instituto Nacional de Câncer José Alencar Gomes da Silva/ Ministério da Saúde (Brasil). Atlas da Mortalidade. Available at: https://mortalidade.inca.gov.br/MortalidadeWeb/. Accessed 29 Dec 2019.
-
- Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26(35):5802–5812. doi: 10.1200/JCO.2008.16.4368. - DOI - PMC - PubMed