Viral infiltration of pancreatic islets in patients with COVID-19
- PMID: 34112801
- PMCID: PMC8192507
- DOI: 10.1038/s41467-021-23886-3
Viral infiltration of pancreatic islets in patients with COVID-19
Abstract
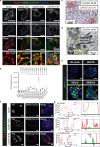
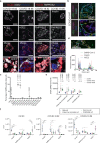
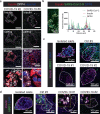
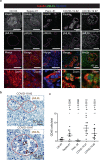
Metabolic diseases are associated with an increased risk of severe COVID-19 and conversely, new-onset hyperglycemia and complications of preexisting diabetes have been observed in COVID-19 patients. Here, we performed a comprehensive analysis of pancreatic autopsy tissue from COVID-19 patients using immunofluorescence, immunohistochemistry, RNA scope and electron microscopy and detected SARS-CoV-2 viral infiltration of beta-cells in all patients. Using SARS-CoV-2 pseudoviruses, we confirmed that isolated human islet cells are permissive to infection. In eleven COVID-19 patients, we examined the expression of ACE2, TMPRSS and other receptors and factors, such as DPP4, HMBG1 and NRP1, that might facilitate virus entry. Whereas 70% of the COVID-19 patients expressed ACE2 in the vasculature, only 30% displayed ACE2-expression in beta-cells. Even in the absence of manifest new-onset diabetes, necroptotic cell death, immune cell infiltration and SARS-CoV-2 viral infection of pancreatic beta-cells may contribute to varying degrees of metabolic dysregulation in patients with COVID-19.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

