High-dose Mycobacterium tuberculosis aerosol challenge cannot overcome BCG-induced protection in Chinese origin cynomolgus macaques; implications of natural resistance for vaccine evaluation
- PMID: 34112845
- PMCID: PMC8192909
- DOI: 10.1038/s41598-021-90913-0
High-dose Mycobacterium tuberculosis aerosol challenge cannot overcome BCG-induced protection in Chinese origin cynomolgus macaques; implications of natural resistance for vaccine evaluation
Abstract
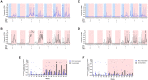
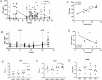
This study describes the use of cynomolgus macaques of Chinese origin (CCM) to evaluate the efficacy and immunogenicity of the BCG vaccine against high dose aerosol Mycobacterium tuberculosis challenge. Progressive disease developed in three of the unvaccinated animals within 10 weeks of challenge, whereas all six vaccinated animals controlled disease for 26 weeks. Three unvaccinated animals limited disease progression, highlighting the intrinsic ability of this macaque species to control disease in comparison to macaques of other species and genotypes. Low levels of IFNγ were induced by BCG vaccination in CCM suggesting that IFNγ alone does not provide a sufficiently sensitive biomarker of vaccination in this model. An early response after challenge, together with the natural bias towards terminal effector memory T-cell populations and the contribution of monocytes appears to enhance the ability of CCM to naturally control infection. The high dose aerosol challenge model of CCM has value for examination of the host immune system to characterise control of infection which would influence future vaccine design. Although it may not be the preferred platform for the assessment of prophylactic vaccine candidates, the model could be well suited for testing post-exposure vaccination strategies and drug evaluation studies.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- World Health Organization. Global tuberculosis report 2019. https://apps.who.int/iris/handle/10665/329368. License: CC BY-NC-SA 3.0 IGO (2019)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

