I(nsp1)ecting SARS-CoV-2-ribosome interactions
- PMID: 34112887
- PMCID: PMC8192748
- DOI: 10.1038/s42003-021-02265-0
I(nsp1)ecting SARS-CoV-2-ribosome interactions
Abstract
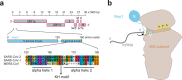
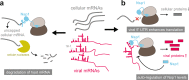
While SARS-CoV-2 is causing modern human history's most serious health crisis and upending our way of life, clinical and basic research on the virus is advancing rapidly, leading to fascinating discoveries. Two studies have revealed how the viral virulence factor, nonstructural protein 1 (Nsp1), binds human ribosomes to inhibit host cell translation. Here, we examine the main conclusions on the molecular activity of Nsp1 and its role in suppressing innate immune responses. We discuss different scenarios potentially explaining how the viral RNA can bypass its own translation blockage and speculate on the suitability of Nsp1 as a therapeutic target.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/director-general/speeches/detail/who-director-genera... (2020).
-
- WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int (2020).
-
- Phylogeny of SARS-like betacoronaviruses including novel coronavirus SARS-CoV-2. https://nextstrain.org/groups/blab/sars-like-cov (2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

