SARS-CoV-2 serology in 4000 health care and administrative staff across seven sites in Lombardy, Italy
- PMID: 34112899
- PMCID: PMC8192543
- DOI: 10.1038/s41598-021-91773-4
SARS-CoV-2 serology in 4000 health care and administrative staff across seven sites in Lombardy, Italy
Abstract
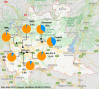
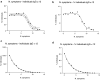
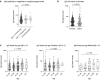
Lombardy is the Italian region most affected by COVID-19. We tested the presence of plasma anti-SARS-CoV-2 IgG antibodies in 3985 employees across 7 healthcare facilities in areas of Lombardy with different exposure to the SARS-CoV-2 epidemic. Subjects filled a questionnaire to self-report on COVID-19 symptoms, comorbidities, smoking, regular or remote working, and the exposure to COVID-infected individuals. We show that the number of individuals exposed to the virus depended on the geographical location of the facility, ranging between 3 and 43%, consistent with the spatial variation of COVID-19 incidence in Lombardy, and correlated with family interactions. We observed a higher prevalence of females than males positive for IgG, however the level of antibodies was similar, suggesting a comparable magnitude of the anti-spike antibody response. IgG positivity among smokers was lower (7.4% vs 13.5%) although without difference in IgG plasma levels. We observed 11.9% of IgG positive asymptomatic individuals and another 23.1% with one or two symptoms. Interestingly, among the IgG positive population, 81.2% of subjects with anosmia/dysgeusia and fever were SARS-CoV-2 infected, indicating that these symptoms are strongly associated to COVID-19. In conclusion, the frequency of IgG positivity and SARS-CoV-2 infection is dependent on the geographical exposure to the virus and primarily to family rather than hospital exposure.
Conflict of interest statement
The authors declare no competing interests.
Figures
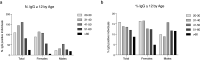

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

