Heterozygous loss-of-function variants significantly expand the phenotypes associated with loss of GDF11
- PMID: 34113007
- PMCID: PMC8487929
- DOI: 10.1038/s41436-021-01216-8
Heterozygous loss-of-function variants significantly expand the phenotypes associated with loss of GDF11
Abstract
Purpose: Growth differentiation factor 11 (GDF11) is a key signaling protein required for proper development of many organ systems. Only one prior study has associated an inherited GDF11 variant with a dominant human disease in a family with variable craniofacial and vertebral abnormalities. Here, we expand the phenotypic spectrum associated with GDF11 variants and document the nature of the variants.
Methods: We present a cohort of six probands with de novo and inherited nonsense/frameshift (4/6 patients) and missense (2/6) variants in GDF11. We generated gdf11 mutant zebrafish to model loss of gdf11 phenotypes and used an overexpression screen in Drosophila to test variant functionality.
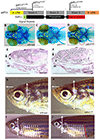
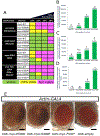
Results: Patients with variants in GDF11 presented with craniofacial (5/6), vertebral (5/6), neurological (6/6), visual (4/6), cardiac (3/6), auditory (3/6), and connective tissue abnormalities (3/6). gdf11 mutant zebrafish show craniofacial abnormalities and body segmentation defects that match some patient phenotypes. Expression of the patients' variants in the fly showed that one nonsense variant in GDF11 is a severe loss-of-function (LOF) allele whereas the missense variants in our cohort are partial LOF variants.
Conclusion: GDF11 is needed for human development, particularly neuronal development, and LOF GDF11 alleles can affect the development of numerous organs and tissues.
© 2021. The Author(s), under exclusive licence to the American College of Medical Genetics and Genomics.
Conflict of interest statement
Declaration of interests
The Department of Molecular and Human Genetics at Baylor College of Medicine receives revenue from clinical genetic testing conducted at Baylor Genetics Laboratories. The authors have no other conflicts of interest.
Figures


References
-
- Lee SJ (1990). Identification of a novel member (GDF-1) of the transforming growth factor-β superfamily. - PubMed
-
- Frikha R (2020). Klippel-Feil syndrome: a review of the literature. Clin. Dysmorphol 29, 35–37. - PubMed
-
- Naikmasur VG, Sattur AP, Kirty RN, and Thakur AR (2011). Type III Klippel-Feil syndrome: Case report and review of associated craniofacial anomalies. Odontology 99, 197–202. - PubMed
-
- Asai-Coakwell M, French CR, Ye M, Garcha K, Bigot K, Perera AG, Staehling-Hampton K, Mema SC, Chanda B, Mushegian A, et al. (2009). Incomplete penetrance and phenotypic variability characterize Gdf6-attributable oculo-skeletal phenotypes. Hum. Mol. Genet 18, 1110–1121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

