An Inhaled PI3Kδ Inhibitor Improves Recovery in Acutely Exacerbating COPD Patients: A Randomized Trial
- PMID: 34113093
- PMCID: PMC8184151
- DOI: 10.2147/COPD.S309129
An Inhaled PI3Kδ Inhibitor Improves Recovery in Acutely Exacerbating COPD Patients: A Randomized Trial
Abstract
Purpose: This study evaluated the safety and efficacy of inhaled nemiralisib, a phosphoinositide 3-kinase δ (PI3Kδ) inhibitor, in patients with an acute exacerbation of chronic obstructive pulmonary disease (COPD).
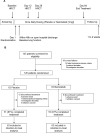
Methods: In this double-blind, placebo-controlled study, 126 patients (40-80 years with a post-bronchodilator forced expiratory volume in 1 sec (FEV1) ≤80% of predicted (previously documented)) were randomized 1:1 to once daily inhaled nemiralisib (1 mg) or placebo for 84 days, added to standard of care. The primary endpoint was specific imaging airway volume (siVaw) after 28 treatment days and was analyzed using a Bayesian repeated measures model (clintrials.gov: NCT02294734).
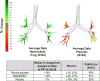
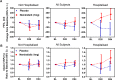
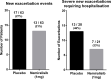
Results: A total of 126 patients were randomized to treatment; 55 on active treatment and 49 on placebo completed the study. When comparing nemiralisib and placebo-treated patients, an 18% placebo-corrected increase from baseline in distal siVaw (95% credible intervals (Cr I) (-1%, 42%)) was observed on Day 28. The probability that the true treatment ratio was >0% (Pr(θ>0)) was 96%, suggestive of a real treatment effect. Improvements were observed across all lung lobes. Patients treated with nemiralisib experienced a 107.3 mL improvement in posterior median FEV1 (change from baseline, 95% Cr I (-2.1, 215.5)) at day 84, compared with placebo. Adverse events were reported by 41 patients on placebo and 49 on nemiralisib, the most common being post-inhalation cough on nemiralisib (35%) vs placebo (3%).
Conclusion: These data show that addition of nemiralisib to usual care delivers more effective recovery from an acute exacerbation and improves lung function parameters including siVaw and FEV1. Although post-inhalation cough was identified, nemiralisib was otherwise well tolerated, providing a promising novel therapy for this acutely ill patient group.
Keywords: COPD; acute exacerbation; nemiralisib.
© 2021 Cahn et al.
Conflict of interest statement
AC, JR, MB, GD, CL, YC, MMiz, MMont and EJ are GSK employees and all hold GSK shares. JNH, RW and EMH were GSK employees at the time of the study conduct, analysis and interpretation, and hold GSK shares. JNH and EMH are named on patents for compound GSK2269557 (nemiralisib). JNH reports patents WO2010125082A1 and WO2012055846A1 issued. EMH has a patent WO2015055691A1 issued. CVH, WV and JDB are employees of FLUIDDA, the company responsible for FRI analysis and interpretation. WV and JDB hold FLUIDDA shares. WDB reports costs from University Hospital Antwerp to perform the study. The current affiliation of JNH is Discovery, Charles River, Chesterford Research Park, Cambridge, UK. The current affiliation of RW is DLRC, Letchworth Garden City, UK. The current affiliation of WV is OncoRadiomics, Liège, Belgium. The current affiliation of EMH is Eligo Bioscience, Paris, France. The authors report no other conflicts of interest in this work.
Figures

References
-
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2020. Available from: http://goldcopd.org/. Accessed May6, 2021.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

