The Effect of Viloxazine Extended-Release Capsules on Functional Impairments Associated with Attention-Deficit/Hyperactivity Disorder (ADHD) in Children and Adolescents in Four Phase 3 Placebo-Controlled Trials
- PMID: 34113106
- PMCID: PMC8184252
- DOI: 10.2147/NDT.S312011
The Effect of Viloxazine Extended-Release Capsules on Functional Impairments Associated with Attention-Deficit/Hyperactivity Disorder (ADHD) in Children and Adolescents in Four Phase 3 Placebo-Controlled Trials
Abstract
Purpose: The ADHD Rating Scale (ADHD-RS) assesses 18 symptoms of inattention and hyperactivity/impulsivity and has been used in many clinical trials to evaluate the treatment effect of drugs on ADHD. The fifth edition of this scale (ADHD-RS-5) also assesses the impact of inattention and hyperactivity/impulsivity symptoms on six domains of functional impairment (FI): family relationships, peer relationships, completing/returning homework, academic performance at school, controlling behavior at school, and self-esteem. Here, we report the effect of viloxazine extended-release capsules (viloxazine ER), a novel nonstimulant treatment for ADHD in children and adolescents (ages 6-17 years), on FI from a post hoc analysis of four randomized, double-blind, placebo-controlled Phase 3 clinical trials (N=1354).
Patients and methods: ADHD-RS-5 investigator ratings of ADHD symptoms and FIs were conducted at baseline and weekly post-baseline for 6-8 weeks in the four trials. Change from baseline (CFB) in ADHD-RS-5 FI scores (Total score [sum of 12 FI items] and Inattention and Hyperactivity/Impulsivity subscale scores [sum of 6 corresponding FI items]) and the 30% and 50% Responder Rates (ADHD-RS-5 FI Total score) were compared between viloxazine ER and placebo.
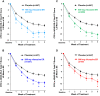
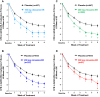
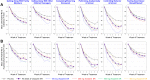
Results: The reduction (improvement) in ADHD-RS-5 FI scores (Total and subscale scores) and the percentage of responders (30% and 50%) at Week 6 were significantly greater in each viloxazine ER dose group vs placebo. In the 100-400 mg/day viloxazine ER groups, improvements were found as early as Week 1 (100-mg/day) or Week 2 (200-, 400-mg/day) of treatment. Analysis of individual items of ADHD-related FIs demonstrated that the effect of viloxazine ER was observed across all domains of impairment.
Conclusion: Significant improvements observed in ADHD-related FIs are consistent with the reduction in inattention and hyperactivity/impulsivity symptoms demonstrated in the viloxazine ER Phase 3 pediatric trials. Therefore, viloxazine ER provides clinically meaningful improvement of ADHD symptoms and functioning in children and adolescents with ADHD, starting as early as Week 1-2 of treatment.
Keywords: academic performance; behavior; impairment domains; self-esteem.
© 2021 Nasser et al.
Conflict of interest statement
AN, JTH, TL, GDB, ZM, and JR are employees of Supernus Pharmaceuticals, Inc. FAL has served as a consultant to and received speaker fees and/or research support from Eli Lilly, GSK, Ironshore, Neos, Novartis, Noven, Pfizer, Shire, Sunovion, Supernus, and Tris. ACC has served as a consultant, participated in advisory board meetings and received speaker fees and/or research support from Allergan, Takeda (Shire), Emalex, Akili, Arbor, Cingulate, Ironshore, Aevi Genomic Medicine, Neos, Otsuka, Pfizer, Purdue, Adlon, Rhodes, Sunovion, Tris, KemPharm, Supernus, US FDA, Corium, Jazz, Tulex Pharma, and Receptor. The authors report no other conflicts of interest in this work.
Figures

References
-
- Faraone SV, Asherson P, Banaschewski T, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. 2015;1:15020. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

