Kynurenines and Neurofilament Light Chain in Multiple Sclerosis
- PMID: 34113231
- PMCID: PMC8185147
- DOI: 10.3389/fnins.2021.658202
Kynurenines and Neurofilament Light Chain in Multiple Sclerosis
Abstract
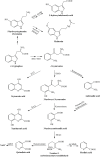
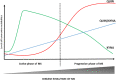
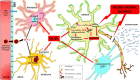
Multiple sclerosis is an autoimmune, demyelinating, and neurodegenerative disease of the central nervous system. In recent years, it has been proven that the kynurenine system plays a significant role in the development of several nervous system disorders, including multiple sclerosis. Kynurenine pathway metabolites have both neurotoxic and neuroprotective effects. Moreover, the enzymes of the kynurenine pathway play an important role in immunomodulation processes, among others, as well as interacting with neuronal energy balance and various redox reactions. Dysregulation of many of the enzymatic steps in kynurenine pathway and upregulated levels of these metabolites locally in the central nervous system, contribute to the progression of multiple sclerosis pathology. This process can initiate a pathogenic cascade, including microglia activation, glutamate excitotoxicity, chronic oxidative stress or accumulated mitochondrial damage in the axons, that finally disrupt the homeostasis of neurons, leads to destabilization of neuronal cell cytoskeleton, contributes to neuro-axonal damage and neurodegeneration. Neurofilaments are good biomarkers of the neuro-axonal damage and their level reliably indicates the severity of multiple sclerosis and the treatment response. There is increasing evidence that connections exist between the molecules generated in the kynurenine metabolic pathway and the change in neurofilament concentrations. Thus the alterations in the kynurenine pathway may be an important biomarker of the course of multiple sclerosis. In our present review, we report the possible relationship and connection between neurofilaments and the kynurenine system in multiple sclerosis based on the available evidences.
Keywords: glutamate excitotoxicity; kynurenine; mitochondrial dysfunction; multiple sclerosis; neuro-axonal damage; neurofilaments; oxidative stress; quinolinic acid.
Copyright © 2021 Pukoli, Polyák, Rajda and Vécsei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Anderson J. M., Hampton D. W., Patani R., Pryce G., Crowther R. A., Reynolds R., et al. (2008). Abnormally phosphorylated tau is associated with neuronal and axonal loss in experimental autoimmune encephalomyelitis and multiple sclerosis. Brain 131 (Pt 7) 1736–1748. 10.1093/brain/awn119 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

