Effectiveness and Overall Safety of NutropinAq® for Growth Hormone Deficiency and Other Paediatric Growth Hormone Disorders: Completion of the International Cooperative Growth Study, NutropinAq® European Registry (iNCGS)
- PMID: 34113318
- PMCID: PMC8185283
- DOI: 10.3389/fendo.2021.676083
Effectiveness and Overall Safety of NutropinAq® for Growth Hormone Deficiency and Other Paediatric Growth Hormone Disorders: Completion of the International Cooperative Growth Study, NutropinAq® European Registry (iNCGS)
Abstract
Objective: The International Cooperative Growth Study, NutropinAq® European Registry (iNCGS) (NCT00455728) monitored long-term safety and effectiveness of recombinant human growth hormone (rhGH; NutropinAq® [somatropin]) in paediatric growth disorders.
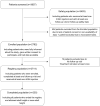
Methods: Open-label, non-interventional, post-marketing surveillance study recruiting children with growth disorders. Endpoints included gain in height standard deviation score (SDS), adult height, and occurrence of adverse events (AEs).
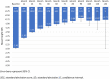
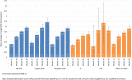
Results: 2792 patients were enrolled. 2082 patients (74.6%) had growth hormone deficiency (GHD), which was isolated idiopathic in 1825 patients (87.7%). Non-GHD diagnoses included Turner syndrome (TS) (n=199), chronic renal insufficiency (CRI) (n=10), other non-GHD (n=498), and missing data for three participants. Improvements from baseline height SDS occurred at all time points to Month 132, and in all subgroups by disease aetiology. At Month 12, mean (95% CI) change in height SDS by aetiology was: idiopathic GHD 0.63 (0.61;0.66), organic GHD 0.71 (0.62;0.80), TS 0.59 (0.53; 0.65), CRI 0.54 (-0.49;1.56), and other non-GHD 0.64 (0.59;0.69). Mean height ( ± SD) at the last visit among the 235 patients with adult or near-adult height recorded was 154.0 cm ( ± 8.0) for girls and 166.7 cm ( ± 8.0) for boys. The most frequent biological and clinical non-serious drug-related AEs were increased insulin-like growth factor concentrations (314 events) and injection site haematoma (99 events). Serious AEs related to rhGH according to investigators were reported (n=30); the most frequent were scoliosis (4 events), epiphysiolysis (3 events), and strabismus (2 events).
Conclusions: There was an improvement in mean height SDS in all aetiology subgroups after rhGH treatment. No new safety concerns were identified.
Keywords: NutropinAq® (somatropin); growth hormone deficiency; paediatric GH disorders; rhGH, recombinant human GH; safety.
Copyright © 2021 Coutant, Bosch Muñoz, Dumitrescu, Schnabel, Sert, Perrot and Dattani.
Conflict of interest statement
RC received honoraria for lectures and for the scientific organization of meetings from Sandoz, Ipsen, Novo Nordisk, Lilly, and Pfizer. JB-M received honoraria for lectures from Lilly, Merck Ferring, Sandoz and Ipsen and participated in Ipsen advisory board. DS served in advisory boards for Kyowa Kirin, Ipsen, Merck Serono, Novo Nordisk; received honoraria for lectures and for the scientific organization of meetings from, Hexal/Sandoz Ipsen, Merck Serono, Novo Nordisk and Pfizer; and received research grants from Hexal/Sandoz, Kyowa Kirin and Pfizer. MD received honoraria for lectures and for the scientific organization of meetings from Sandoz, Ipsen, Novo Nordisk and Pfizer. CS and VP are employees of Ipsen. The authors declare that this study received funding from Ipsen. The funder contributed to study design, data collection and analysis, decision to publish, and funded editorial support for preparation of the manuscript. The authors take full responsibility for the entire content of this submitted manuscript and approved submission. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Ten years with biosimilar rhGH in clinical practice in Sweden - experience from the prospective PATRO children and adult studies.BMC Endocr Disord. 2020 Apr 29;20(1):55. doi: 10.1186/s12902-020-0535-4. BMC Endocr Disord. 2020. PMID: 32349731 Free PMC article.
-
Safety and Effectiveness of a Biosimilar Recombinant Growth Hormone in Adults with Growth Hormone Deficiency: Analysis of Final Data from PATRO Adults, an International Post-Marketing Surveillance Study.Drug Des Devel Ther. 2024 Dec 5;18:5729-5741. doi: 10.2147/DDDT.S471967. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39659948 Free PMC article.
-
Safety and Effectiveness of Omnitrope®, a Biosimilar Recombinant Human Growth Hormone: More Than 10 Years' Experience from the PATRO Children Study.Horm Res Paediatr. 2020;93(3):154-163. doi: 10.1159/000508190. Epub 2020 Aug 19. Horm Res Paediatr. 2020. PMID: 32814319 Clinical Trial.
-
Observed and predicted total pubertal growth during treatment with growth hormone in adolescents with idiopathic growth hormone deficiency, Turner syndrome, short stature, born small for gestational age and idiopathic short stature: KIGS analysis and review.Horm Res Paediatr. 2011;75(6):423-32. doi: 10.1159/000324117. Epub 2011 Feb 25. Horm Res Paediatr. 2011. PMID: 21358173 Review.
-
Use of growth hormone in children.Nat Clin Pract Endocrinol Metab. 2006 May;2(5):260-8. doi: 10.1038/ncpendmet0169. Nat Clin Pract Endocrinol Metab. 2006. PMID: 16932297 Review.
Cited by
-
Effectiveness and Safety of Combination Therapy with Herbal Medicine and Growth Hormone Compared to Growth Hormone Monotherapy for Short Stature Children: A Systematic Review and Meta-Analysis.Evid Based Complement Alternat Med. 2022 Aug 9;2022:5725258. doi: 10.1155/2022/5725258. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35982997 Free PMC article. Review.
-
Long-term Pegylated GH for Children With GH Deficiency: A Large, Prospective, Real-world Study.J Clin Endocrinol Metab. 2023 Jul 14;108(8):2078-2086. doi: 10.1210/clinem/dgad039. J Clin Endocrinol Metab. 2023. PMID: 36669772 Free PMC article.
-
Safety and Effectiveness of a Biosimilar Recombinant Human Growth Hormone in Children Requiring Growth Hormone Treatment: Analysis of Final Data from PATRO Children, an International, Post-Marketing Surveillance Study.Drug Des Devel Ther. 2024 Mar 2;18:667-684. doi: 10.2147/DDDT.S440009. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38454934 Free PMC article.
-
Factors Associated With Response to Growth Hormone in Pediatric Growth Disorders: Results of a 5-year Registry Analysis.J Endocr Soc. 2023 Feb 16;7(5):bvad026. doi: 10.1210/jendso/bvad026. eCollection 2023 Mar 6. J Endocr Soc. 2023. PMID: 36936713 Free PMC article.
-
The association between idiopathic scoliosis and growth hormone treatment in short children.Ann Pediatr Endocrinol Metab. 2022 Sep;27(3):207-213. doi: 10.6065/apem.2142186.093. Epub 2022 May 16. Ann Pediatr Endocrinol Metab. 2022. PMID: 35592900 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

