Cellulolytic and Xylanolytic Microbial Communities Associated With Lignocellulose-Rich Wheat Straw Degradation in Anaerobic Digestion
- PMID: 34113323
- PMCID: PMC8186499
- DOI: 10.3389/fmicb.2021.645174
Cellulolytic and Xylanolytic Microbial Communities Associated With Lignocellulose-Rich Wheat Straw Degradation in Anaerobic Digestion
Abstract
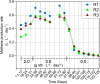
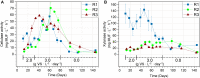
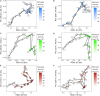
The enzymatic hydrolysis of lignocellulosic polymers is generally considered the rate-limiting step to methane production in anaerobic digestion of lignocellulosic biomass. The present study aimed to investigate how the hydrolytic microbial communities of three different types of anaerobic digesters adapted to lignocellulose-rich wheat straw in continuous stirred tank reactors operated for 134 days. Cellulase and xylanase activities were monitored weekly using fluorescently-labeled model substrates and the enzymatic profiles were correlated with changes in microbial community compositions based on 16S rRNA gene amplicon sequencing to identify key species involved in lignocellulose degradation. The enzymatic activity profiles and microbial community changes revealed reactor-specific adaption of phylogenetically different hydrolytic communities. The enzymatic activities correlated significantly with changes in specific taxonomic groups, including representatives of Ruminiclostridium, Caldicoprobacter, Ruminofilibacter, Ruminococcaceae, Treponema, and Clostridia order MBA03, all of which have been linked to cellulolytic and xylanolytic activity in the literature. By identifying microorganisms with similar development as the cellulase and xylanase activities, the proposed correlation method constitutes a promising approach for deciphering essential cellulolytic and xylanolytic microbial groups for anaerobic digestion of lignocellulosic biomass.
Keywords: anaerobic digestion; biogas; fluorometric enzyme assay; hydrolysis; lignocellulose; microbial adaptation; microbial community; wheat straw.
Copyright © 2021 Jensen, de Jonge, Dolriis, Kragelund, Fischer, Eskesen, Noer, Møller, Ottosen, Nielsen and Kofoed.
Conflict of interest statement
NJ is employed by the company NIRAS A/S. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Effects of different pretreatment methods on biogas production and microbial community in anaerobic digestion of wheat straw.Environ Sci Pollut Res Int. 2021 Oct;28(37):51772-51785. doi: 10.1007/s11356-021-14296-5. Epub 2021 May 15. Environ Sci Pollut Res Int. 2021. PMID: 33990921
-
Enhanced biomethane production rate and yield from lignocellulosic ensiled forage ley by in situ anaerobic digestion treatment with endogenous cellulolytic enzymes.Biotechnol Biofuels. 2017 May 16;10:129. doi: 10.1186/s13068-017-0814-0. eCollection 2017. Biotechnol Biofuels. 2017. PMID: 28523077 Free PMC article.
-
Effect of bioaugmentation by cellulolytic bacteria enriched from sheep rumen on methane production from wheat straw.Anaerobe. 2017 Aug;46:122-130. doi: 10.1016/j.anaerobe.2017.03.013. Epub 2017 Mar 18. Anaerobe. 2017. PMID: 28323135
-
Biomethane Production From Lignocellulose: Biomass Recalcitrance and Its Impacts on Anaerobic Digestion.Front Bioeng Biotechnol. 2019 Aug 8;7:191. doi: 10.3389/fbioe.2019.00191. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31440504 Free PMC article. Review.
-
Application of next-generation sequencing methods for microbial monitoring of anaerobic digestion of lignocellulosic biomass.Appl Microbiol Biotechnol. 2017 Sep;101(18):6849-6864. doi: 10.1007/s00253-017-8438-7. Epub 2017 Aug 4. Appl Microbiol Biotechnol. 2017. PMID: 28779289 Review.
Cited by
-
Multivariate comparison of taxonomic, chemical and operational data from 80 different full-scale anaerobic digester-related systems.Biotechnol Biofuels Bioprod. 2024 Jun 20;17(1):84. doi: 10.1186/s13068-024-02525-1. Biotechnol Biofuels Bioprod. 2024. PMID: 38902807 Free PMC article.
-
Evaluating the Role of Postbiotics in the Modulation of Human Oral Microbiota: A Randomized Controlled Clinical Trial.Probiotics Antimicrob Proteins. 2024 Mar 19. doi: 10.1007/s12602-024-10238-y. Online ahead of print. Probiotics Antimicrob Proteins. 2024. PMID: 38502383
-
Host Lifeform Shapes Phyllospheric Microbiome Assembly in Mountain Lake: Deterministic Selection and Stochastic Colonization Dynamics.Microorganisms. 2025 Apr 23;13(5):960. doi: 10.3390/microorganisms13050960. Microorganisms. 2025. PMID: 40431132 Free PMC article.
-
Thermophilic Hemicellulases Secreted by Microbial Consortia Selected from an Anaerobic Digester.Int J Mol Sci. 2024 Sep 13;25(18):9887. doi: 10.3390/ijms25189887. Int J Mol Sci. 2024. PMID: 39337375 Free PMC article.
-
Energetically exploiting lignocellulose-rich residues in anaerobic digestion technologies: from bioreactors to proteogenomics.Biotechnol Biofuels Bioprod. 2023 Nov 28;16(1):183. doi: 10.1186/s13068-023-02432-x. Biotechnol Biofuels Bioprod. 2023. PMID: 38017526 Free PMC article.
References
-
- Achinas S., Achinas V., Euverink G. J. W. (2017). A technological overview of biogas production from biowaste. Engineering 3 299–307. 10.1016/J.ENG.2017.03.002 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

