When the Good Syndrome Goes Bad: A Systematic Literature Review
- PMID: 34113351
- PMCID: PMC8185358
- DOI: 10.3389/fimmu.2021.679556
When the Good Syndrome Goes Bad: A Systematic Literature Review
Abstract
Background: Good syndrome is a rare adult-onset immunodeficiency characterized by thymoma and hypogammaglobulinemia. Its clinical manifestations are highly heterogeneous, ranging from various infections to autoimmunity.
Objective: This study was to summarize patient characteristics, identify prognostic factors and define clinical subgroups of Good syndrome.
Methods: A systematic literature review was conducted to include patients with Good syndrome identified in PubMed, Embase and Cochrane databases between January 2010 and November 2020. Logistic and Cox regressions were used to identify prognostic factors impacting outcomes. Clinical subgroups were defined by multiple correspondence analysis and unsupervised hierarchical clustering. A decision tree was constructed to characterize the subgroup placement of cases.
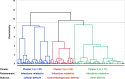
Results: Of 162 patients included in the current study, the median age at diagnosis was 58 years and 51% were male. Type AB was the most common histological subtype of thymoma, and infections as well as concurrent autoimmune disorders were identified in 92.6% and 51.2% patients, respectively. Laboratory workup showed typical findings of combined immunodeficiency. Thymoma status (odds ratio [OR] 4.157, confidence interval [CI] 1.219-14.177, p = 0.023), infections related to cellular immunity defects (OR 3.324, 95% CI 1.100-10.046, p = 0.033), infections of sinopulmonary tract (OR 14.351, 95% CI 2.525-81.576, p = 0.003), central nerve system (OR 6.403, 95% CI 1.205-34.027, p = 0.029) as well as bloodstream (OR 6.917, 95% CI 1.519-31.505, p = 0.012) were independent prognostic factors. The 10-year overall survival was 53.7%. Cluster analysis revealed three clinical subgroups with distinct characteristics and prognosis (cluster 1, infections related to cellular immunity defects; cluster 2, infections related to other immunity defects; cluster 3, infections related to humoral and phagocytic immunity defects). A decision tree using infection types (related to humoral and cellular immunity defects) could place patients into corresponding clusters with an overall correct prediction of 72.2%.
Conclusions: Infection type and site were the main prognostic factors impacting survival of patients with Good syndrome. We identified three subgroups within Good syndrome associated with distinct clinical features, which may facilitate the study of underlying pathogenesis as well as development of targeted therapy.
Keywords: Good syndrome; clinical subgroups; immunodeficiency; infections; prognosis.
Copyright © 2021 Shi and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

