Run for your life: can exercise be used to effectively target GLUT4 in diabetic cardiac disease?
- PMID: 34113491
- PMCID: PMC8162245
- DOI: 10.7717/peerj.11485
Run for your life: can exercise be used to effectively target GLUT4 in diabetic cardiac disease?
Abstract
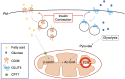
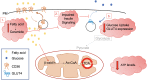
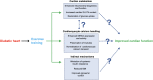
The global incidence, associated mortality rates and economic burden of diabetes are now such that it is considered one of the most pressing worldwide public health challenges. Considerable research is now devoted to better understanding the mechanisms underlying the onset and progression of this disease, with an ultimate aim of improving the array of available preventive and therapeutic interventions. One area of particular unmet clinical need is the significantly elevated rate of cardiomyopathy in diabetic patients, which in part contributes to cardiovascular disease being the primary cause of premature death in this population. This review will first consider the role of metabolism and more specifically the insulin sensitive glucose transporter GLUT4 in diabetic cardiac disease, before addressing how we may use exercise to intervene in order to beneficially impact key functional clinical outcomes.
Keywords: Cardiomyocyte; Diabetic cardiomyopathy; Exercise; GLUT4; Glucose.
© 2021 Bowman et al.
Conflict of interest statement
Gwyn W. Gould is an Academic Editor for PeerJ. There are no other competing interests.
Figures

Similar articles
-
Tuberculosis.In: Holmes KK, Bertozzi S, Bloom BR, Jha P, editors. Major Infectious Diseases. 3rd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2017 Nov 3. Chapter 11. In: Holmes KK, Bertozzi S, Bloom BR, Jha P, editors. Major Infectious Diseases. 3rd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2017 Nov 3. Chapter 11. PMID: 30212088 Free Books & Documents. Review.
-
Targeted GLUT-4 deficiency in the heart induces cardiomyocyte hypertrophy and impaired contractility linked with Ca(2+) and proton flux dysregulation.J Mol Cell Cardiol. 2010 Apr;48(4):663-72. doi: 10.1016/j.yjmcc.2009.11.017. Epub 2009 Dec 3. J Mol Cell Cardiol. 2010. PMID: 19962383
-
Caragana jubata ethanol extract ameliorates the symptoms of STZ-HFD-induced T2DM mice by PKC/GLUT4 pathway.J Ethnopharmacol. 2025 Jan 13;339:119171. doi: 10.1016/j.jep.2024.119171. Epub 2024 Nov 28. J Ethnopharmacol. 2025. PMID: 39613004
-
Exercise training increases insulin-stimulated glucose disposal and GLUT4 (SLC2A4) protein content in patients with type 2 diabetes.Diabetologia. 2006 Dec;49(12):2983-92. doi: 10.1007/s00125-006-0457-3. Epub 2006 Sep 26. Diabetologia. 2006. PMID: 17019595
-
Exercise and diabetes.Cardiol Clin. 2001 Aug;19(3):489-505. doi: 10.1016/s0733-8651(05)70231-9. Cardiol Clin. 2001. PMID: 11570119 Review.
Cited by
-
The potential role of miR-27a and miR-320a in metabolic syndrome in obese Egyptian females.J Genet Eng Biotechnol. 2022 May 19;20(1):75. doi: 10.1186/s43141-022-00348-x. J Genet Eng Biotechnol. 2022. PMID: 35590121 Free PMC article.
-
Identifying and establishing the critical elements of a human cardiac in-vitro model for studying type-II diabetes.Discov Appl Sci. 2025;7(7):788. doi: 10.1007/s42452-025-07442-y. Epub 2025 Jul 15. Discov Appl Sci. 2025. PMID: 40678470 Free PMC article.
-
Exercise medicine for cancer cachexia: targeted exercise to counteract mechanisms and treatment side effects.J Cancer Res Clin Oncol. 2022 Jun;148(6):1389-1406. doi: 10.1007/s00432-022-03927-0. Epub 2022 Jan 27. J Cancer Res Clin Oncol. 2022. PMID: 35088134 Free PMC article. Review.
-
Impact of cancer cachexia on respiratory muscle function and the therapeutic potential of exercise.J Physiol. 2022 Dec;600(23):4979-5004. doi: 10.1113/JP283569. Epub 2022 Nov 1. J Physiol. 2022. PMID: 36251564 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

