Prophylaxis and treatment of influenza: options, antiviral susceptibility, and existing recommendations
- PMID: 34113534
- PMCID: PMC8165743
- DOI: 10.3205/id000071
Prophylaxis and treatment of influenza: options, antiviral susceptibility, and existing recommendations
Abstract
Influenza viruses of types A and B attack 5-10% of adults and 20-30% of children, thereby causing millions of acute respiratory infections in Germany annually. A significant number of these infections are associated with complications such as pneumonia and bacterial superinfections that need hospitalization and might lead to death. In addition to vaccines, drugs were developed that might support influenza prevention and that can be used to treat influenza patients. The timely application of anti-influenza drugs can inhibit virus replication, help reduce and shorten the symptoms, and prevent death as well as virus transmission. This review concisely describes the mechanism of action, the potential for prophylactic and therapeutic use, and the knowledge on resistance of anti-influenza drugs approved today. However, the main aim is to give an overview on the recommendations available in Germany for the proper use of these drugs. In doing so, the recommendations published in statements and guidelines of medical societies as well as the German influenza pandemic preparedness plan are summarized with the consideration of specific circumstances and groups of patients.
Keywords: baloxavir; infection; neuraminidase; polymerase; resistance.
Copyright © 2021 Duwe et al.
Conflict of interest statement
SD, BS, JT, OA, HF and MS declare that they have no competing interests. BG received fees for talks and/or advisory board: Roche, Seqirus, Sanofi, GSK.
Figures
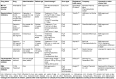


References
-
- Robert Koch-Institut. Bericht zur Epidemiologie der Influenza in Deutschland Saison 2017/18. Berlin: RKI; 2018. - DOI
-
- Robert Koch-Institut. Bericht zur Epidemiologie der Influenza in Deutschland Saison 2018/19. Berlin: RKI; 2019. - DOI
-
- Robert Koch-Institut. Influenza (Teil 1): Erkrankungen durch saisonale Influenzaviren – RKI-Ratgeber. Berlin: RKI; 2016. [last updated 2016 Feb 12]. Available from: https://www.rki.de/DE/Content/Infekt/EpidBull/Merkblaetter/Ratgeber_Infl....
-
- Suess T, Remschmidt C, Schink S, Luchtenberg M, Haas W, Krause G, Buchholz U. Facemasks and intensified hand hygiene in a German household trial during the 2009/2010 influenza A(H1N1) pandemic: adherence and tolerability in children and adults. Epidemiol Infect. 2011 Dec;139(12):1895–1901. doi: 10.1017/S0950268810003006. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
