Understanding the Impact of Uterine Fibroids on Human Endometrium Function
- PMID: 34113609
- PMCID: PMC8186666
- DOI: 10.3389/fcell.2021.633180
Understanding the Impact of Uterine Fibroids on Human Endometrium Function
Abstract
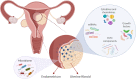
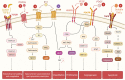
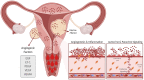
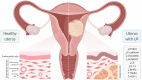
Uterine fibroids (leiomyomas) are the most common benign gynecological tumors in women of reproductive age worldwide. They cause heavy menstrual bleeding, usually leading to severe anemia, pelvic pain/pressure, infertility, and other debilitating morbidities. Fibroids are believed to be monoclonal tumors arising from the myometrium, and recent studies have demonstrated that fibroids actively influence the endometrium globally. Studies suggest a direct relationship between the number of fibroids removed and fertility problems. In this review, our objective was to provide a complete overview of the origin of uterine fibroids and the molecular pathways and processes implicated in their development and growth, which can directly affect the function of a healthy endometrium. One of the most common characteristics of fibroids is the excessive production of extracellular matrix (ECM) components, which contributes to the stiffness and expansion of fibroids. ECM may serve as a reservoir of profibrotic growth factors such as the transforming growth factor β (TGF-β) and a modulator of their availability and actions. Fibroids also elicit mechanotransduction changes that result in decreased uterine wall contractility and increased myometrium rigidity, which affect normal biological uterine functions such as menstrual bleeding, receptivity, and implantation. Changes in the microRNA (miRNA) expression in fibroids and myometrial cells appear to modulate the TGF-β pathways and the expression of regulators of ECM production. Taken together, these findings demonstrate an interaction among the ECM components, TGF-β family signaling, miRNAs, and the endometrial vascular system. Targeting these components will be fundamental to developing novel pharmacotherapies that not only treat uterine fibroids but also restore normal endometrial function.
Keywords: endometrial receptivity; endometrium; heavy menstrual bleeding; implantation; subfertility; transforming growth factor beta; uterine fibroids.
Copyright © 2021 Navarro, Bariani, Yang and Al-Hendy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Increased expression of neurogenic factors in uterine fibroids.Hum Reprod. 2019 Nov 1;34(11):2153-2162. doi: 10.1093/humrep/dez182. Hum Reprod. 2019. PMID: 31732726
-
Literature Review on the Role of Uterine Fibroids in Endometrial Function.Reprod Sci. 2018 May;25(5):635-643. doi: 10.1177/1933719117725827. Epub 2017 Aug 22. Reprod Sci. 2018. PMID: 28826369 Free PMC article. Review.
-
Mechanism of Endometrial Receptivity Affected by Fibroids.Am J Reprod Immunol. 2024 Dec;92(6):e70022. doi: 10.1111/aji.70022. Am J Reprod Immunol. 2024. PMID: 39625040 Free PMC article. Review.
-
Adenomyosis pathogenesis: insights from next-generation sequencing.Hum Reprod Update. 2021 Oct 18;27(6):1086-1097. doi: 10.1093/humupd/dmab017. Hum Reprod Update. 2021. PMID: 34131719 Free PMC article. Review.
-
Endometrial vascular development in heavy menstrual bleeding: altered spatio-temporal expression of endothelial cell markers and extracellular matrix components.Hum Reprod. 2018 Mar 1;33(3):399-410. doi: 10.1093/humrep/dex378. Hum Reprod. 2018. PMID: 29309596
Cited by
-
Biomechanical Forces Determine Fibroid Stem Cell Transformation and the Receptivity Status of the Endometrium: A Critical Appraisal.Int J Mol Sci. 2022 Nov 17;23(22):14201. doi: 10.3390/ijms232214201. Int J Mol Sci. 2022. PMID: 36430682 Free PMC article. Review.
-
Cyclical endometrial repair and regeneration: Molecular mechanisms, diseases, and therapeutic interventions.MedComm (2020). 2023 Dec 1;4(6):e425. doi: 10.1002/mco2.425. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 38045828 Free PMC article. Review.
-
Cyclic processes in the uterine tubes, endometrium, myometrium, and cervix: pathways and perturbations.Mol Hum Reprod. 2023 Apr 29;29(5):gaad012. doi: 10.1093/molehr/gaad012. Mol Hum Reprod. 2023. PMID: 37225518 Free PMC article. Review.
-
Hypoxia in uterine fibroids: role in pathobiology and therapeutic opportunities.Oxygen (Basel). 2024 Jun;4(2):236-252. doi: 10.3390/oxygen4020013. Epub 2024 May 28. Oxygen (Basel). 2024. PMID: 38957794 Free PMC article.
-
Assessing the Hepatic Safety of Epigallocatechin Gallate (EGCG) in Reproductive-Aged Women.Nutrients. 2023 Jan 9;15(2):320. doi: 10.3390/nu15020320. Nutrients. 2023. PMID: 36678191 Free PMC article. Clinical Trial.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

