The Epithelial-to-Mesenchymal Transition (EMT) in Development and Cancer
- PMID: 34113749
- PMCID: PMC8189433
- DOI: 10.1146/annurev-cancerbio-030518-055425
The Epithelial-to-Mesenchymal Transition (EMT) in Development and Cancer
Abstract
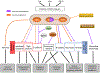
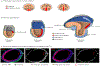
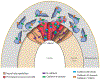
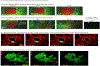
Epithelial-to-mesenchymal transitions (EMTs) are complex cellular processes where cells undergo dramatic changes in signaling, transcriptional programming, and cell shape, while directing the exit of cells from the epithelium and promoting migratory properties of the resulting mesenchyme. EMTs are essential for morphogenesis during development and are also a critical step in cancer progression and metastasis formation. Here we provide an overview of the molecular regulation of the EMT process during embryo development, focusing on chick and mouse gastrulation and neural crest development. We go on to describe how EMT regulators participate in the progression of pancreatic and breast cancer in mouse models, and discuss the parallels with developmental EMTs and how these help to understand cancer EMTs. We also highlight the differences between EMTs in tumor and in development to arrive at a broader view of cancer EMT. We conclude by discussing how further advances in the field will rely on in vivo dynamic imaging of the cellular events of EMT.
Keywords: Epithelial-to-Mesenchymal Transition; breast cancer; cancer progression; gastrulation; intravital imaging; pancreatic ductal adenocarcinoma.
Figures




References
-
- Acloque H, Ocana OH, Abad D, Stern CD, Nieto MA. 2017. Snail2 and Zeb2 repress P-cadherin to define embryonic territories in the chick embryo. Development 144:649–56 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
