Mechanisms of typhoid toxin neutralization by antibodies targeting glycan receptor binding and nuclease subunits
- PMID: 34113815
- PMCID: PMC8169802
- DOI: 10.1016/j.isci.2021.102454
Mechanisms of typhoid toxin neutralization by antibodies targeting glycan receptor binding and nuclease subunits
Abstract
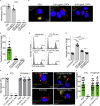
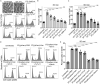
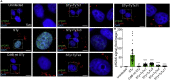
Nearly all clinical isolates of Salmonella Typhi, the cause of typhoid fever, are antibiotic resistant. All S. Typhi isolates secrete an A2B5 exotoxin called typhoid toxin to benefit the pathogen during infection. Here, we demonstrate that antibiotic-resistant S. Typhi secretes typhoid toxin continuously during infection regardless of antibiotic treatment. We characterize typhoid toxin antibodies targeting glycan-receptor-binding PltB or nuclease CdtB, which neutralize typhoid toxin in vitro and in vivo, as demonstrated by using typhoid toxin secreted by antibiotic-resistant S. Typhi during human cell infection and lethal dose typhoid toxin challenge to mice. TyTx11 generated in this study neutralizes typhoid toxin effectively, comparable to TyTx4 that binds to all PltB subunits available per holotoxin. Cryoelectron microscopy explains that the binding of TyTx11 to CdtB makes this subunit inactive through CdtB catalytic-site conformational change. The identified toxin-neutralizing epitopes are conserved across all S. Typhi clinical isolates, offering critical insights into typhoid toxin-neutralizing strategies.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Neutralization of Typhoid Toxin by Alpaca-Derived, Single-Domain Antibodies Targeting the PltB and CdtB Subunits.Infect Immun. 2022 Feb 17;90(2):e0051521. doi: 10.1128/IAI.00515-21. Epub 2021 Dec 13. Infect Immun. 2022. PMID: 34898253 Free PMC article.
-
Molecular Insights into the Assembly and Functional Diversification of Typhoid Toxin.mBio. 2022 Feb 22;13(1):e0191621. doi: 10.1128/mbio.01916-21. Epub 2022 Jan 11. mBio. 2022. PMID: 35012347 Free PMC article.
-
Alternate typhoid toxin assembly evolved independently in the two Salmonella species.mBio. 2024 Apr 10;15(4):e0340323. doi: 10.1128/mbio.03403-23. Epub 2024 Mar 19. mBio. 2024. PMID: 38501873 Free PMC article.
-
The Role of Typhoid Toxin in Salmonella Typhi Virulence .Yale J Biol Med. 2017 Jun 23;90(2):283-290. eCollection 2017 Jun. Yale J Biol Med. 2017. PMID: 28656014 Free PMC article. Review.
-
Emerging insights into the biology of typhoid toxin.Curr Opin Microbiol. 2017 Feb;35:70-77. doi: 10.1016/j.mib.2017.01.012. Epub 2017 Feb 16. Curr Opin Microbiol. 2017. PMID: 28213043 Free PMC article. Review.
Cited by
-
Global Genomic Characterization of Salmonella enterica Serovar Telelkebir.Front Microbiol. 2021 Jul 29;12:704152. doi: 10.3389/fmicb.2021.704152. eCollection 2021. Front Microbiol. 2021. PMID: 34394052 Free PMC article.
-
Single missense mutations in Vi capsule synthesis genes confer hypervirulence to Salmonella Typhi.Nat Commun. 2024 Jun 19;15(1):5258. doi: 10.1038/s41467-024-49590-6. Nat Commun. 2024. PMID: 38898034 Free PMC article.
-
The structural basis of Salmonella A2B5 toxin neutralization by antibodies targeting the glycan-receptor binding subunits.Cell Rep. 2021 Sep 7;36(10):109654. doi: 10.1016/j.celrep.2021.109654. Cell Rep. 2021. PMID: 34496256 Free PMC article.
-
Complete Genome Sequence of Salmonella enterica Serovar Typhi Strain ISP2825.Microbiol Resour Announc. 2021 Oct 14;10(41):e0080421. doi: 10.1128/MRA.00804-21. Epub 2021 Oct 14. Microbiol Resour Announc. 2021. PMID: 34647800 Free PMC article.
-
Glycan-mediated molecular interactions in bacterial pathogenesis.Trends Microbiol. 2022 Mar;30(3):254-267. doi: 10.1016/j.tim.2021.06.011. Epub 2021 Jul 14. Trends Microbiol. 2022. PMID: 34274195 Free PMC article. Review.
References
-
- Abrahams G.L., Hensel M. Manipulating cellular transport and immune responses: dynamic interactions between intracellular Salmonella enterica and its host cells. Cell Microbiol. 2006;8:728–737. - PubMed
-
- CDC . Oxford University Press; 2017. CDC Yellow Book 2020: Health Information for International Travel. Chapter 4. Travel-related infectious diseases.
Grants and funding
LinkOut - more resources
Full Text Sources

