Diverse meibum lipids produced by Awat1 and Awat2 are important for stabilizing tear film and protecting the ocular surface
- PMID: 34113821
- PMCID: PMC8169949
- DOI: 10.1016/j.isci.2021.102478
Diverse meibum lipids produced by Awat1 and Awat2 are important for stabilizing tear film and protecting the ocular surface
Erratum in
-
Erratum: Diverse meibum lipids produced by Awat1 and Awat2 are important for stabilizing tear film and protecting the ocular surface.iScience. 2021 Sep 25;24(10):103160. doi: 10.1016/j.isci.2021.103160. eCollection 2021 Oct 22. iScience. 2021. PMID: 34622178 Free PMC article.
Abstract
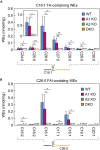
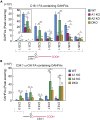
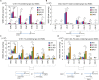
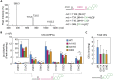
A lipid layer consisting of meibum lipids exists in the tear film and functions in preventing dry eye disease. Although the meibum lipids include diverse lipid classes, the synthesis pathway and role of each class remain largely unknown. Here, we created single and double knockout (KO and DKO, respectively) mice for the two acyl-CoA wax alcohol acyltransferases (Awat1 and Awat2) and investigated their dry eye phenotypes and meibum lipid composition. Awat2 KO and DKO mice exhibited severe dry eye with meibomian gland dysfunction, whereas Awat1 KO mice had mild dry eye. In these mice, specific meibum lipid classes were reduced: (O-acyl)-ω-hydroxy fatty acids and type 1ω wax diesters in Awat1 KO mice, wax monoesters and types 1ω and 2ω wax diesters in Awat2 KO mice, and most of these in DKO mice. Our findings reveal that Awat1 and Awat2 show characteristic substrate specificity and together produce diverse meibum lipids.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Meibomian gland lipid alterations and ocular surface sequela in Awat2 knockout murine model of meibomian gland dysfunction and evaporative dry eye disease.Ocul Surf. 2024 Oct;34:489-503. doi: 10.1016/j.jtos.2024.10.003. Epub 2024 Oct 15. Ocul Surf. 2024. PMID: 39414024 Free PMC article.
-
Formation of fatty alcohols-components of meibum lipids-by the fatty acyl-CoA reductase FAR2 is essential for dry eye prevention.FASEB J. 2022 Apr;36(4):e22216. doi: 10.1096/fj.202101733R. FASEB J. 2022. PMID: 35238077
-
Deficiency in Acyl-CoA:Wax Alcohol Acyltransferase 2 causes evaporative dry eye disease by abolishing biosynthesis of wax esters.FASEB J. 2020 Oct;34(10):13792-13808. doi: 10.1096/fj.202001191R. Epub 2020 Aug 26. FASEB J. 2020. PMID: 32851726 Free PMC article.
-
Analysis of meibum and tear lipids.Ocul Surf. 2012 Oct;10(4):230-50. doi: 10.1016/j.jtos.2012.07.004. Epub 2012 Jul 25. Ocul Surf. 2012. PMID: 23084145 Review.
-
The presence and significance of polar meibum and tear lipids.Ocul Surf. 2015 Jan;13(1):26-42. doi: 10.1016/j.jtos.2014.06.002. Epub 2014 Oct 8. Ocul Surf. 2015. PMID: 25557344 Review.
Cited by
-
Acyl-CoA:wax alcohol acyltransferase 2 modulates the cone visual cycle in mouse retina.FASEB J. 2022 Jul;36(7):e22390. doi: 10.1096/fj.202101855RRR. FASEB J. 2022. PMID: 35665537 Free PMC article.
-
Comparative Biophysical Study of Meibomian Lipids of Wild Type and Soat1-Null Mice: Implications to Meibomian Gland Dysfunction and Dry Eye Disease.Invest Ophthalmol Vis Sci. 2023 Aug 1;64(11):20. doi: 10.1167/iovs.64.11.20. Invest Ophthalmol Vis Sci. 2023. PMID: 37585190 Free PMC article.
-
Expression of Acyl-CoA wax-alcohol acyltransferase 2 (AWAT2) by human and rabbit meibomian glands and meibocytes.Ocul Surf. 2022 Jan;23:60-70. doi: 10.1016/j.jtos.2021.11.010. Epub 2021 Nov 24. Ocul Surf. 2022. PMID: 34838721 Free PMC article.
-
Meibomian gland lipid alterations and ocular surface sequela in Awat2 knockout murine model of meibomian gland dysfunction and evaporative dry eye disease.Ocul Surf. 2024 Oct;34:489-503. doi: 10.1016/j.jtos.2024.10.003. Epub 2024 Oct 15. Ocul Surf. 2024. PMID: 39414024 Free PMC article.
-
Development and validation of a method to generate phenol red thread tests.Ocul Surf. 2024 Oct;34:262-266. doi: 10.1016/j.jtos.2024.08.007. Epub 2024 Aug 8. Ocul Surf. 2024. PMID: 39127389 Free PMC article.
References
-
- Bron A.J., de Paiva C.S., Chauhan S.K., Bonini S., Gabison E.E., Jain S., Knop E., Markoulli M., Ogawa Y., Perez V. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017;15:438–510. - PubMed
-
- Bron A.J., Tiffany J.M., Gouveia S.M., Yokoi N., Voon L.W. Functional aspects of the tear film lipid layer. Exp. Eye Res. 2004;78:347–360. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

