Technology development of hyperthermic pressurized intraperitoneal aerosol chemotherapy (hPIPAC)
- PMID: 34114069
- PMCID: PMC8523399
- DOI: 10.1007/s00464-021-08567-y
Technology development of hyperthermic pressurized intraperitoneal aerosol chemotherapy (hPIPAC)
Abstract
Background: Optimized drug delivery systems are needed for intraperitoneal chemotherapy. The aim of this study was to develop a technology for applying pressurized intraperitoneal aerosol chemotherapy (PIPAC) under hyperthermic conditions (hPIPAC).
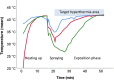
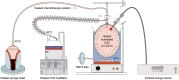
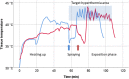
Methods: This is an ex-vivo study in an inverted bovine urinary bladder (IBUB). Hyperthermia was established using a modified industry-standard device (Humigard). Two entry and one exit ports were placed. Warm-humid CO2 was insufflated in the IBUB placed in a normothermic bath to simulate body thermal inertia. The temperature of the aerosol, tissue, and water bath was measured in real-time.
Results: Therapeutic hyperthermia (target tissue temperature 41-43 °C) could be established and maintained over 30 min. In the first phase (insufflation phase), tissue hyperthermia was created by insufflating continuously warm-humid CO2. In the second phase (aerosolization phase), chemotherapeutic drugs were heated up and aerosolized into the IBUB. In a third phase (application phase), hyperthermia was maintained within the therapeutic range using an endoscopic infrared heating device. In a fourth phase, the toxic aerosol was discarded using a closed aerosol waste system (CAWS).
Discussion: We introduce a simple and effective technology for hPIPAC. hPIPAC is feasible in an ex-vivo model by using a combination of industry-standard medical devices after modification. Potential pharmacological and biological advantages of hPIPAC over PIPAC should now be evaluated.
Keywords: Aerosol; Hyperthermia; Intraperitoneal chemotherapy; Laparoscopy; Medical devices.
© 2021. The Author(s).
Conflict of interest statement
MAR is the holder of several patents on PIPAC technology and shareholder of Capnomed GmbH, Villingendorf, Germany. The other authors have no potential conflict of interest to disclose.
Figures
Similar articles
-
Hyperthermic pressurized intraperitoneal aerosol drug delivery system in a large animal model: a feasibility and safety study.Surg Endosc. 2024 Apr;38(4):2062-2069. doi: 10.1007/s00464-024-10702-4. Epub 2024 Mar 1. Surg Endosc. 2024. PMID: 38429574
-
Efficacy of Hyperthermic Pressurized Intraperitoneal Aerosol Chemotherapy in an In Vitro Model Using a Human Gastric Cancer AGS Cell Line and an Abdominal Cavity Model.J Gastric Cancer. 2024 Jul;24(3):246-256. doi: 10.5230/jgc.2024.24.e24. J Gastric Cancer. 2024. PMID: 38960884 Free PMC article.
-
Feasibility of hyperthermic pressurized intraperitoneal aerosol chemotherapy in a porcine model.Surg Endosc. 2016 Oct;30(10):4258-64. doi: 10.1007/s00464-015-4738-0. Epub 2015 Dec 29. Surg Endosc. 2016. PMID: 26715024
-
Pressurized Intraperitoneal Aerosol Chemotherapy, a Palliative Treatment Approach for Patients With Peritoneal Carcinomatosis: Description of Method and Systematic Review of Literature.Dis Colon Rectum. 2020 Feb;63(2):242-255. doi: 10.1097/DCR.0000000000001565. Dis Colon Rectum. 2020. PMID: 31914116
-
Overcoming Drug Resistance by Taking Advantage of Physical Principles: Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC).Cancers (Basel). 2019 Dec 20;12(1):34. doi: 10.3390/cancers12010034. Cancers (Basel). 2019. PMID: 31877647 Free PMC article. Review.
Cited by
-
Effects of Hyperthermia and Hyperthermic Intraperitoneal Chemoperfusion on the Peritoneal and Tumor Immune Contexture.Cancers (Basel). 2023 Aug 29;15(17):4314. doi: 10.3390/cancers15174314. Cancers (Basel). 2023. PMID: 37686590 Free PMC article. Review.
-
Design of Magnetic Hydrogels for Hyperthermia and Drug Delivery.Polymers (Basel). 2021 Dec 4;13(23):4259. doi: 10.3390/polym13234259. Polymers (Basel). 2021. PMID: 34883761 Free PMC article. Review.
-
Emerging trends of the tumor microenvironment in peritoneal malignancies (2010-2024): a visualization analysis.Front Oncol. 2025 Jun 4;15:1515476. doi: 10.3389/fonc.2025.1515476. eCollection 2025. Front Oncol. 2025. PMID: 40535136 Free PMC article.
-
Is PIPAC a Treatment Option in Upper and Lower Gastrointestinal Cancer with Peritoneal Metastasis?Visc Med. 2022 Apr;38(2):90-98. doi: 10.1159/000523901. Epub 2022 Mar 21. Visc Med. 2022. PMID: 35614892 Free PMC article. Review.
-
Amplifying Curcumin's Antitumor Potential: A Heat-Driven Approach for Colorectal Cancer Treatment.Onco Targets Ther. 2024 Jan 30;17:63-78. doi: 10.2147/OTT.S448024. eCollection 2024. Onco Targets Ther. 2024. PMID: 38313386 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

