Apigenin restores endothelial function by ameliorating oxidative stress, reverses aortic stiffening, and mitigates vascular inflammation with aging
- PMID: 34114892
- PMCID: PMC8321807
- DOI: 10.1152/ajpheart.00118.2021
Apigenin restores endothelial function by ameliorating oxidative stress, reverses aortic stiffening, and mitigates vascular inflammation with aging
Abstract
We assessed the efficacy of oral supplementation with the flavanoid apigenin on arterial function during aging and identified critical mechanisms of action. Young (6 mo) and old (27 mo) C57BL/6N mice (model of arterial aging) consumed drinking water containing vehicle (0.2% carboxymethylcellulose; 10 young and 7 old) or apigenin (0.5 mg/mL in vehicle; 10 young and 9 old) for 6 wk. In vehicle-treated animals, isolated carotid artery endothelium-dependent dilation (EDD), bioassay of endothelial function, was impaired in old versus young (70% ± 9% vs. 92% ± 1%, P < 0.0001) due to reduced nitric oxide (NO) bioavailability. Old mice had greater arterial reactive oxygen species (ROS) production and oxidative stress (higher nitrotyrosine) associated with greater nicotinamide adenine dinucleotide phosphate oxidase (oxidant enzyme) and lower superoxide dismutase 1 and 2 (antioxidant enzymes); ex vivo administration of Tempol (antioxidant) restored EDD to young levels, indicating ROS-mediated suppression of EDD. Old animals also had greater aortic stiffness as indicated by higher aortic pulse wave velocity (PWV, 434 ± 9 vs. 346 ± 5 cm/s, P < 0.0001) due to greater intrinsic aortic wall stiffness associated with lower elastin levels and higher collagen, advanced glycation end products (AGEs), and proinflammatory cytokine abundance. In old mice, apigenin restored EDD (96% ± 2%) by increasing NO bioavailability, normalized arterial ROS, oxidative stress, and antioxidant expression, and abolished ROS inhibition of EDD. Moreover, apigenin prevented foam cell formation in vitro (initiating step in atherosclerosis) and mitigated age-associated aortic stiffening (PWV 373 ± 5 cm/s) by normalizing aortic intrinsic wall stiffness, collagen, elastin, AGEs, and inflammation. Thus, apigenin is a promising therapeutic for arterial aging.NEW & NOTEWORTHY Our study provides novel evidence that oral apigenin supplementation can reverse two clinically important indicators of arterial dysfunction with age, namely, vascular endothelial dysfunction and large elastic artery stiffening, and prevents foam cell formation in an established cell culture model of early atherosclerosis. Importantly, our results provide extensive insight into the biological mechanisms of apigenin action, including increased nitric oxide bioavailability, normalization of age-related increases in arterial ROS production and oxidative stress, reversal of age-associated aortic intrinsic mechanical wall stiffening and adverse remodeling of the extracellular matrix, and suppression of vascular inflammation. Given that apigenin is commercially available as a dietary supplement in humans, these preclinical findings provide the experimental basis for future translational studies assessing the potential of apigenin to treat arterial dysfunction and reduce cardiovascular disease risk with aging.
Keywords: aortic pulse wave velocity; atherosclerosis; endothelium-dependent dilation; nutraceutical; reactive oxygen species.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




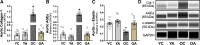

References
-
- Brunt VE, Gioscia-Ryan RA, Richey JJ, Zigler MC, Cuevas LM, Gonzalez A, Vázquez-Baeza Y, Battson ML, Smithson AT, Gilley AD, Ackermann G, Neilson AP, Weir T, Davy KP, Knight R, Seals DR. Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J Physiol 597: 2361–2378, 2019. doi:10.1113/JP277336. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

