TDP-43 stabilizes G3BP1 mRNA: relevance to amyotrophic lateral sclerosis/frontotemporal dementia
- PMID: 34115105
- PMCID: PMC8677511
- DOI: 10.1093/brain/awab217
TDP-43 stabilizes G3BP1 mRNA: relevance to amyotrophic lateral sclerosis/frontotemporal dementia
Abstract
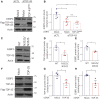
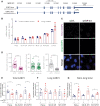
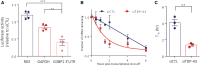
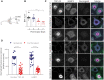
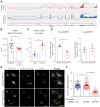
TDP-43 nuclear depletion and concurrent cytoplasmic accumulation in vulnerable neurons is a hallmark feature of progressive neurodegenerative proteinopathies such as amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). Cellular stress signalling and stress granule dynamics are now recognized to play a role in ALS/FTD pathogenesis. Defective stress granule assembly is associated with increased cellular vulnerability and death. Ras-GAP SH3-domain-binding protein 1 (G3BP1) is a critical stress granule assembly factor. Here, we define that TDP-43 stabilizes G3BP1 transcripts via direct binding of a highly conserved cis regulatory element within the 3' untranslated region. Moreover, we show in vitro and in vivo that nuclear TDP-43 depletion is sufficient to reduce G3BP1 protein levels. Finally, we establish that G3BP1 transcripts are reduced in ALS/FTD patient neurons bearing TDP-43 cytoplasmic inclusions/nuclear depletion. Thus, our data indicate that, in ALS/FTD, there is a compromised stress granule response in disease-affected neurons due to impaired G3BP1 mRNA stability caused by TDP-43 nuclear depletion. These data implicate TDP-43 and G3BP1 loss of function as contributors to disease.
Keywords: G3BP1; TDP-43; amyotrophic lateral sclerosis; frontotemporal dementia; stress granules.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


References
-
- Mompean M, Romano V, Pantoja-Uceda D, et al.The TDP-43 N-terminal domain structure at high resolution. Febs J. 2016;283(7):1242–60. - PubMed
-
- Neumann M, Sampathu DM, Kwong LK, et al.Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–133. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

