CXCL2-CXCR2 axis mediates αV integrin-dependent peritoneal metastasis of colon cancer cells
- PMID: 34115261
- PMCID: PMC8318971
- DOI: 10.1007/s10585-021-10103-0
CXCL2-CXCR2 axis mediates αV integrin-dependent peritoneal metastasis of colon cancer cells
Erratum in
-
Correction: CXCL2-CXCR2 axis mediates αV integrin-dependent peritoneal metastasis of colon cancer cells.Clin Exp Metastasis. 2024 Oct;41(5):813-814. doi: 10.1007/s10585-024-10302-5. Clin Exp Metastasis. 2024. PMID: 39066931 Free PMC article. No abstract available.
Abstract
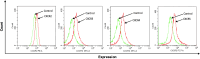
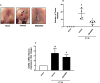
Peritoneal metastasis is an insidious aspect of colorectal cancer. The aim of the present study was to define mechanisms regulating colon cancer cell adhesion and spread to peritoneal wounds after abdominal surgery. Mice was laparotomized and injected intraperitoneally with CT-26 colon carcinoma cells and metastatic noduli in the peritoneal cavity was quantified after treatment with a CXCR2 antagonist or integrin-αV-antibody. CT-26 cells expressed cell surface chemokine receptors CXCR2, CXCR3, CXCR4 and CXCR5. Stimulation with the CXCR2 ligand, CXCL2, dose-dependently increased proliferation and migration of CT-26 cells in vitro. The CXCR2 antagonist, SB225002, dose-dependently decreased CXCL2-induced proliferation and migration of colon cancer cells in vitro. Intraperitoneal administration of CT-26 colon cancer cells resulted in wide-spread growth of metastatic nodules at the peritoneal surface of laparotomized animals. Laparotomy increased gene expression of CXCL2 at the incisional line. Pretreatment with CXCR2 antagonist reduced metastatic nodules by 70%. Moreover, stimulation with CXCL2 increased CT-26 cell adhesion to extracellular matrix (ECM) proteins in a CXCR2-dependent manner. CT-26 cells expressed the αV, β1 and β3 integrin subunits and immunoneutralization of αV abolished CXCL2-triggered adhesion of CT-26 to vitronectin, fibronectin and fibrinogen. Finally, inhibition of the αV integrin significantly attenuated the number of carcinomatosis nodules by 69% in laparotomized mice. These results were validated by use of the human colon cancer cell line HT-29 in vitro. Our data show that colon cancer cell adhesion and growth on peritoneal wound sites is mediated by a CXCL2-CXCR2 signaling axis and αV integrin-dependent adhesion to ECM proteins.
Keywords: Chemokines; Chemotaxis; Integrins; Metastasis; Peritoneal carcinomatosis.
© 2021. The Author(s).
Conflict of interest statement
Authors have no financial conflicts of interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

