Preclinical Safety of a 3D-Printed Hydroxyapatite-Demineralized Bone Matrix Scaffold for Spinal Fusion
- PMID: 34115714
- PMCID: PMC8765284
- DOI: 10.1097/BRS.0000000000004142
Preclinical Safety of a 3D-Printed Hydroxyapatite-Demineralized Bone Matrix Scaffold for Spinal Fusion
Abstract
Study design: Prospective, randomized, controlled preclinical study.
Objective: The objective of this study was to compare the host inflammatory response of our previously described hyperelastic, 3D-printed (3DP) hydroxyapatite (HA)-demineralized bone matrix (DBM) composite scaffold to the response elicited with the use of recombinant human bone morphogenetic protein-2 (rhBMP-2) in a preclinical rat posterolateral lumbar fusion model.
Summary of background data: Our group previously found that this 3D-printed HA-DBM composite material shows promise as a bone graft substitute in a preclinical rodent model, but its safety profile had yet to be assessed.
Methods: Sixty female Sprague-Dawley rats underwent bilateral posterolateral intertransverse lumbar spinal fusion using with the following implants: 1) type I absorbable collagen sponge (ACS) alone; 2) 10 μg rhBMP-2/ACS; or 3) the 3DP HA-DBM composite scaffold (n = 20). The host inflammatory response was assessed using magnetic resonance imaging, while the local and circulating cytokine expression levels were evaluated by enzyme-linked immunosorbent assays at subsequent postoperative time points (N = 5/time point).
Results: At both 2 and 5 days postoperatively, treatment with the HA-DBM scaffold produced significantly less soft tissue edema at the fusion bed site relative to rhBMP-2-treated animals as quantified on magnetic resonance imaging. At every postoperative time point evaluated, the level of soft tissue edema in HA-DBM-treated animals was comparable to that of the ACS control group. At 2 days postoperatively, serum concentrations of tumor necrosis factor-α and macrophage chemoattractant protein-1 were significantly elevated in the rhBMP-2 treatment group relative to ACS controls, whereas these cytokines were not elevated in the HA-DBM-treated animals.
Conclusion: The 3D-printed HA-DBM composite induces a significantly reduced host inflammatory response in a preclinical spinal fusion model relative to rhBMP-2.Level of Evidence: N/A.
Copyright © 2021 Wolters Kluwer Health, Inc. All rights reserved.
Figures


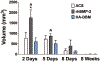


References
-
- Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am 1991;73:802–8. - PubMed
-
- Resnick DK, Watters WC 3rd, Sharan A, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 9: lumbar fusion for stenosis with spondylolisthesis. J Neurosurg Spine 2014;21:54–61. - PubMed
-
- Martin BI, Mirza SK, Spina N, et al. Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004 to 2015. Spine (Phila Pa 1976) 2019;44:369–76. - PubMed
-
- Makino T, Kaito T, Fujiwara H, et al. Does fusion status after posterior lumbar interbody fusion affect patient-based QOL outcomes? An evaluation performed using a patient-based outcome measure. J Orthop Sci 2014;19:707–12. - PubMed
-
- Makino T, Kaito T, Fujiwara H, et al. Risk factors for poor patient-reported quality of life outcomes after posterior lumbar interbody fusion: an analysis of 2-year follow-up. Spine (Phila Pa 1976) 2017;42:1502–10. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

