Experimental transmission of Leishmania (Mundinia) parasites by biting midges (Diptera: Ceratopogonidae)
- PMID: 34115806
- PMCID: PMC8221790
- DOI: 10.1371/journal.ppat.1009654
Experimental transmission of Leishmania (Mundinia) parasites by biting midges (Diptera: Ceratopogonidae)
Abstract
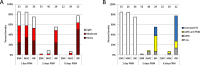
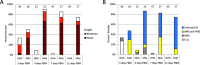
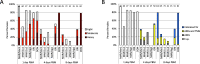
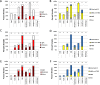
Leishmania parasites, causative agents of leishmaniasis, are currently divided into four subgenera: Leishmania, Viannia, Sauroleishmania and Mundinia. The recently established subgenus Mundinia has a wide geographical distribution and contains five species, three of which have the potential to infect and cause disease in humans. While the other Leishmania subgenera are transmitted exclusively by phlebotomine sand flies (Diptera: Psychodidae), natural vectors of Mundinia remain uncertain. This study investigates the potential of sand flies and biting midges of the genus Culicoides (Diptera: Ceratopogonidae) to transmit Leishmania parasites of the subgenus Mundinia. Sand flies (Phlebotomus argentipes, P. duboscqi and Lutzomyia migonei) and Culicoides biting midges (Culicoides sonorensis) were exposed to five Mundinia species through a chicken skin membrane and dissected at specific time intervals post bloodmeal. Potentially infected insects were also allowed to feed on ear pinnae of anaesthetized BALB/c mice and the presence of Leishmania DNA was subsequently confirmed in the mice using polymerase chain reaction analyses. In C. sonorensis, all Mundinia species tested were able to establish infection at a high rate, successfully colonize the stomodeal valve and produce a higher proportion of metacyclic forms than in sand flies. Subsequently, three parasite species, L. martiniquensis, L. orientalis and L. sp. from Ghana, were transmitted to the host mouse ear by C. sonorensis bite. In contrast, transmission experiments entirely failed with P. argentipes, although colonisation of the stomodeal valve was observed for L. orientalis and L. martiniquensis and metacyclic forms of L. orientalis were recorded. This laboratory-based transmission of Mundinia species highlights that Culicoides are potential vectors of members of this ancestral subgenus of Leishmania and we suggest further studies in endemic areas to confirm their role in the lifecycles of neglected pathogens.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- WHO. Available from: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. 2020.
-
- Muniz J, Medina H. Cutaneous Leishmaniasis in the Guineapig. Hospital (Rio J). 1948;33(1): 7–25. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

