WFDC2 Promotes Spasmolytic Polypeptide-Expressing Metaplasia Through the Up-Regulation of IL33 in Response to Injury
- PMID: 34116028
- PMCID: PMC8380710
- DOI: 10.1053/j.gastro.2021.05.058
WFDC2 Promotes Spasmolytic Polypeptide-Expressing Metaplasia Through the Up-Regulation of IL33 in Response to Injury
Abstract
Background & aims: WAP 4-disulfide core domain protein 2 (WFDC2), also known as human epididymis protein 4, is a small secretory protein that is highly expressed in fibrosis and human cancers, particularly in the ovaries, lungs, and stomach. However, the role of WFDC2 in carcinogenesis is not fully understood. The present study aimed to investigate the role of WFDC2 in gastric carcinogenesis with the use of preneoplastic metaplasia models.
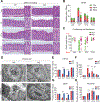
Methods: Three spasmolytic polypeptide-expressing metaplasia (SPEM) models were established in both wild-type and Wfdc2-knockout mice with DMP-777, L635, and high-dose tamoxifen, respectively. To reveal the functional role of WFDC2, we performed transcriptomic analysis with DMP-777-treated gastric corpus specimens.
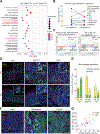
Results: Wfdc2-knockout mice exhibited remarkable resistance against oxyntic atrophy, SPEM emergence, and accumulation of M2-type macrophages in all 3 SPEM models. Transcriptomic analysis revealed that Wfdc2-knockout prevented the up-regulation of interleukin-33 (IL33) expression in the injured mucosal region of SPEM models. Notably, supplementation of recombinant WFDC2 induced IL33 production and M2 macrophage polarization, and ultimately promoted SPEM development. Moreover, long-term treatment with recombinant WFDC2 was able to induce SPEM development.
Conclusions: WFDC2 expressed in response to gastric injury promotes SPEM through the up-regulation of IL33 expression. These findings provide novel insights into the role of WFDC2 in gastric carcinogenesis.
Keywords: Interleukin-33 (IL33); M2 macrophage; SPEM; WFDC2.
Copyright © 2021 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

