The elegant complexity of mammalian ecto-5'-nucleotidase (CD73)
- PMID: 34116887
- PMCID: PMC8448938
- DOI: 10.1016/j.tcb.2021.05.008
The elegant complexity of mammalian ecto-5'-nucleotidase (CD73)
Abstract
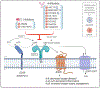
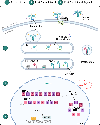
Purinergic signaling is a fundamental mechanism used by all cells to control their internal activities and interact with the environment. A key component of the purinergic system, the enzyme ecto-5'-nucleotidase (CD73) catalyzes the last step in the extracellular metabolism of ATP to form adenosine. Efforts to harness the therapeutic potential of endogenous adenosine in cancer have culminated in the ongoing clinical development of multiple CD73-targeting antibodies and small-molecule inhibitors. However, recent studies are painting an increasingly complex picture of CD73 mRNA and protein regulation and function in cellular homeostasis, physiological adaptation, and disease development. This review discusses the latest conceptual and methodological advances that are helping to unravel the complexity of this important enzyme that was identified nearly 90 years ago.
Keywords: adenosine; cancer; digestive system; inflammation; purinergic system; zonation.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no conflicts of interest.
Figures
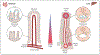

References
-
- Burnstock G (2020) Introduction to purinergic signaling. In Purinergic Signaling, pp. 1–15, Springer. - PubMed
-
- Zimmermann H (2020) History of ectonucleotidases and their role in purinergic signaling. Biochem Pharmacol, 114322. - PubMed
-
- Borea PA et al. (2018) Pharmacology of Adenosine Receptors: The State of the Art. Physiol Rev 98 (3), 1591–1625. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

