High-throughput functional screening for next-generation cancer immunotherapy using droplet-based microfluidics
- PMID: 34117053
- PMCID: PMC8195480
- DOI: 10.1126/sciadv.abe3839
High-throughput functional screening for next-generation cancer immunotherapy using droplet-based microfluidics
Abstract
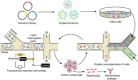
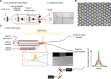
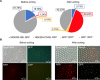
Currently, high-throughput approaches are lacking in the isolation of antibodies with functional readouts beyond simple binding. This situation has impeded the next generation of cancer immunotherapeutics, such as bispecific T cell engager (BiTE) antibodies or agonist antibodies against costimulatory receptors, from reaching their full potential. Here, we developed a highly efficient droplet-based microfluidic platform combining a lentivirus transduction system that enables functional screening of millions of antibodies to identify potential hits with desired functionalities. To showcase the capacity of this system, functional antibodies for CD40 agonism with low frequency (<0.02%) were identified with two rounds of screening. Furthermore, the versatility of the system was demonstrated by combining an anti-Her2 × anti-CD3 BiTE antibody library with functional screening, which enabled efficient identification of active anti-Her2 × anti-CD3 BiTE antibodies. The platform could revolutionize next-generation cancer immunotherapy drug development and advance medical research.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
-
- Andrews L. P., Yano H., Vignali D. A. A., Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: Breakthroughs or backups. Nat. Immunol. 20, 1425–1434 (2019). - PubMed
-
- Tang J., Yu J. X., Hubbard-Lucey V. M., Neftelinov S. T., Hodge J. P., Lin Y., Trial watch: The clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat. Rev. Drug Discov. 17, 854–855 (2018). - PubMed
-
- Hoos A., Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat. Rev. Drug Discov. 15, 235–247 (2016). - PubMed
-
- Mayes P. A., Hance K. W., Hoos A., The promise and challenges of immune agonist antibody development in cancer. Nat. Rev. Drug Discov. 17, 509–527 (2018). - PubMed
-
- Vonderheide R. H., CD40 agonist antibodies in cancer immunotherapy. Annu. Rev. Med. 71, 47–58 (2020). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

