The biological underpinnings of therapeutic resistance in pancreatic cancer
- PMID: 34117095
- PMCID: PMC8247606
- DOI: 10.1101/gad.348523.121
The biological underpinnings of therapeutic resistance in pancreatic cancer
Abstract
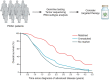
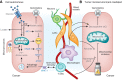
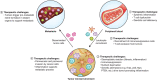
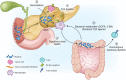
Pancreatic ductal adenocarcinoma (PDAC) is a leading cause of cancer-related mortality in the United States and has only recently achieved a 5-yr survival rate of 10%. This dismal prognosis reflects the remarkable capacity of PDAC to effectively adapt to and resist therapeutic intervention. In this review, we discuss recent advances in our understanding of the biological underpinnings of PDAC and their implications as targetable vulnerabilities in this highly lethal disease.
Keywords: PDAC; genetics; metabolism; microbiome; pancreatic cancer; pancreatic tumor microenvironment; targeted therapy; therapeutic resistance.
© 2021 Beatty et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
References
-
- Aguirre AJ, Nowak JA, Camarda ND, Moffitt RA, Ghazani AA, Hazar-Rethinam M, Raghavan S, Kim J, Brais LK, Ragon D, et al. 2018. Real-time genomic characterization of advanced pancreatic cancer to enable precision medicine. Cancer Discov 8: 1096–1111. 10.1158/2159-8290.CD-18-0275 - DOI - PMC - PubMed
-
- Alcalá S, Sancho P, Martinelli P, Navarro D, Pedrero C, Martín-Hijano L, Valle S, Earl J, Rodríguez-Serrano M, Ruiz-Cañas L, et al. 2020. ISG15 and ISGylation is required for pancreatic cancer stem cell mitophagy and metabolic plasticity. Nat Commun 11: 2682. 10.1038/s41467-020-16395-2 - DOI - PMC - PubMed
-
- Alistar A, Morris BB, Desnoyer R, Klepin HD, Hosseinzadeh K, Clark C, Cameron A, Leyendecker J, D'Agostino R, Topaloglu U, et al. 2017. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol 18: 770–778. 10.1016/S1470-2045(17)30314-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
