Immunogenicity and Safety of a Tetravalent Dengue Vaccine Administered Concomitantly or Sequentially With Tdap Vaccine: Randomized Phase IIIb Trial in Healthy Participants 9-60 Years of Age in the Philippines
- PMID: 34117198
- PMCID: PMC8357045
- DOI: 10.1097/INF.0000000000003220
Immunogenicity and Safety of a Tetravalent Dengue Vaccine Administered Concomitantly or Sequentially With Tdap Vaccine: Randomized Phase IIIb Trial in Healthy Participants 9-60 Years of Age in the Philippines
Abstract
Background: Incorporating dengue vaccination into existing childhood vaccination programs could increase vaccine coverage. This study assessed the safety and immunogenicity of concomitant versus sequential administration of the combined tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine and the tetravalent dengue vaccine (CYD-TDV).
Methods: This phase IIIb, randomized, open-label, multicenter study was conducted in the Philippines in individuals 9-≤60 years of age (NCT02992418). Participants were to receive 3 CYD-TDV doses 6 months apart, the first dose administered either concomitantly or sequentially (28 days post-Tdap). Antibody levels were measured at baseline and 28 days post-first doses of Tdap vaccine and CYD-TDV, using enzyme-linked immunosorbent assay (pertussis, tetanus), micrometabolic inhibition test-toxin neutralization assay (diphtheria) and plaque reduction neutralization test (dengue). Immunogenicity was assessed for all participants, and statistical analysis reported for baseline dengue seropositive participants. Safety was assessed throughout.
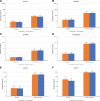
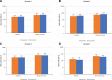
Results: Among 688 randomized participants, 629 (91.4%) were baseline dengue seropositive (concomitant group, n = 314 and sequential group, n = 315). After the first dose, non-inferiority of immune responses between concomitant and sequential vaccination was achieved; between-group geometric mean antibody concentration ratios were close to 1 for anti-PT, anti-FHA, anti-PRN and anti-FIM, between-group differences in percent achieving seroprotection (titers ≥0.1 IU/mL) were 0.26% (diphtheria) and 0.66% (tetanus), and between-group geometric mean antibody titer ratios were close to 1 for dengue serotypes 1-4. Safety profiles in both study groups were comparable.
Conclusions: CYD-TDV and Tdap vaccine administered concomitantly or sequentially in baseline dengue seropositive participants elicited comparable immunogenicity and safety profiles.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
T.C.M., C.V., C.Z., M.-L.T. and S.M. are employees of Sanofi Pasteur and may hold shares and/or stock options in the company. The other authors have no conflicts of interest to disclose.
Figures
References
-
- Jing Q, Wang M. Dengue epidemiology. Glob J Health. 2019;3:37–45.
-
- Pan American Health Organisation. Reported Cases of Dengue Fever in the Americas, 2015-03-11 15:25:43 2020. Available at: https://www.paho.org/data/index.php/en/mnu-topics/indicadores-dengue-en/.... Accessed July 23, 2020.
-
- World Health Organization. Dengue and Severe Dengue, 2020. Available at: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. Accessed June 16, 2020.

