Plasma biomarkers of Alzheimer's disease improve prediction of cognitive decline in cognitively unimpaired elderly populations
- PMID: 34117234
- PMCID: PMC8196018
- DOI: 10.1038/s41467-021-23746-0
Plasma biomarkers of Alzheimer's disease improve prediction of cognitive decline in cognitively unimpaired elderly populations
Abstract
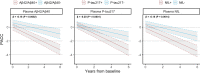
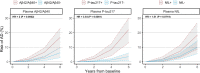
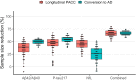
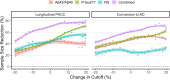
Plasma biomarkers of amyloid, tau, and neurodegeneration (ATN) need to be characterized in cognitively unimpaired (CU) elderly individuals. We therefore tested if plasma measurements of amyloid-β (Aβ)42/40, phospho-tau217 (P-tau217), and neurofilament light (NfL) together predict clinical deterioration in 435 CU individuals followed for an average of 4.8 ± 1.7 years in the BioFINDER study. A combination of all three plasma biomarkers and basic demographics best predicted change in cognition (Pre-Alzheimer's Clinical Composite; R2 = 0.14, 95% CI [0.12-0.17]; P < 0.0001) and subsequent AD dementia (AUC = 0.82, 95% CI [0.77-0.91], P < 0.0001). In a simulated clinical trial, a screening algorithm combining all three plasma biomarkers would reduce the required sample size by 70% (95% CI [54-81]; P < 0.001) with cognition as trial endpoint, and by 63% (95% CI [53-70], P < 0.001) with subsequent AD dementia as trial endpoint. Plasma ATN biomarkers show usefulness in cognitively unimpaired populations and could make large clinical trials more feasible and cost-effective.
Conflict of interest statement
N.C.C., A.L., S.J., S.P., E.S., N.M.C., and A.L.S. report no disclosures. J.L.D. is an employee and stockholder of Eli Lilly and Company. O.H. has acquired research support (for the institution) from AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, GE Healthcare, Pfizer, and Roche. In the past 2 years, he has received consultancy/speaker fees from AC Immune, Alzpath, Biogen, Cerveau and Roche.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

