Feasibility of a home-based foot-ankle exercise programme for musculoskeletal dysfunctions in people with diabetes: randomised controlled FOotCAre (FOCA) Trial II
- PMID: 34117342
- PMCID: PMC8196027
- DOI: 10.1038/s41598-021-91901-0
Feasibility of a home-based foot-ankle exercise programme for musculoskeletal dysfunctions in people with diabetes: randomised controlled FOotCAre (FOCA) Trial II
Abstract
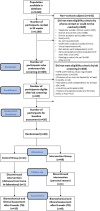
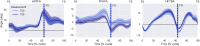
This study sought to assess the feasibility of design, adherence, satisfaction, safety and changes in outcomes followed by a home-based foot-ankle exercise guided by a booklet in individuals with diabetic peripheral neuropathy (DPN). 20 participants were allocated usual care [control group (CG)] or usual care plus home-based foot-ankle exercises [intervention group (IG)] for 8 weeks. For feasibility, we assessed contact, preliminary screening and recruitment rates, adherence, and using a 5-point Likert scale to satisfaction and safety of the booklet. In the IG, we assessed preliminary changes in DPN symptoms, DPN severity (classified by a fuzzy model) and foot-ankle range of motion between baseline and Week 8. In the first 20 weeks, 1310 individuals were screened for eligibility by phone contact. Contact rate was 89% (contacted participants/20w), preliminary screening success 28% (participants underwent screening/20w), and recruitment rate 1.0 participants/week (eligible participants/20w). The recruitment rate was less than the ideal rate of 5 participants/week. The adherence to the exercises programme was 77%, and the dropout was 11% and 9% for the IG and CG, respectively. In the IG, participants' median level of satisfaction was 4 (IQR: 4-5) and perceived safety was 3 (IQR: 3-5). IG significantly decreased the DPN severity (p = 0.020), increased hallux relative to forefoot (first metatarsal) range of motion (ROM) (p < 0.001) and decreased maximum forefoot relative to hindfoot (midfoot motion) dorsiflexion during gait (p = 0.029). The home-based programme was feasible, satisfactory, safe and showed preliminary positive changes in DPN severity and foot motion during gait.Trial Registration ClinicalTrials.gov, NCT04008745. Registered 02/07/2019. https://clinicaltrials.gov/ct2/show/NCT04008745 .
Conflict of interest statement
The authors declare no competing interests.
Figures