The structural basis of fatty acid elongation by the ELOVL elongases
- PMID: 34117479
- PMCID: PMC7611377
- DOI: 10.1038/s41594-021-00605-6
The structural basis of fatty acid elongation by the ELOVL elongases
Abstract
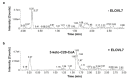
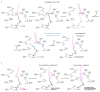
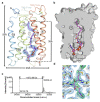
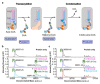
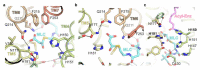
Very long chain fatty acids (VLCFAs) are essential building blocks for the synthesis of ceramides and sphingolipids. The first step in the fatty acid elongation cycle is catalyzed by the 3-keto acyl-coenzyme A (CoA) synthases (in mammals, ELOVL elongases). Although ELOVLs are implicated in common diseases, including insulin resistance, hepatic steatosis and Parkinson's, their underlying molecular mechanisms are unknown. Here we report the structure of the human ELOVL7 elongase, which comprises an inverted transmembrane barrel surrounding a 35-Å long tunnel containing a covalently attached product analogue. The structure reveals the substrate-binding sites in the narrow tunnel and an active site deep in the membrane. We demonstrate that chain elongation proceeds via an acyl-enzyme intermediate involving the second histidine in the canonical HxxHH motif. The unusual substrate-binding arrangement and chemistry suggest mechanisms for selective ELOVL inhibition, relevant for diseases where VLCFAs accumulate, such as X-linked adrenoleukodystrophy.
Conflict of interest statement
The authors declare no competing interests.
Figures

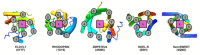





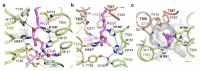

References
-
- Leonard AE, Pereira SL, Sprecher H, Huang Y-S. Elongation of long-chainfatty acids. Progress in Lipid Research. 2004;43:36–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

