Glutathione-Conjugated Hydrogels: Flexible Vehicles for Personalized Treatment of Bacterial Infections
- PMID: 34117588
- PMCID: PMC8351165
- DOI: 10.1007/s11095-021-03057-1
Glutathione-Conjugated Hydrogels: Flexible Vehicles for Personalized Treatment of Bacterial Infections
Abstract
Purpose: Skin and soft tissue infections are increasingly prevalent and often complicated by potentially fatal therapeutic hurdles, such as poor drug perfusion and antibiotic resistance. Delivery vehicles capable of versatile loading may improve local bioavailability and minimize systemic toxicities yet such vehicles are not clinically available. Therefore, we aimed to expand upon the use of glutathione-conjugated poly(ethylene glycol) GSH-PEG hydrogels beyond protein delivery and evaluate the ability to deliver traditional therapeutic molecules.
Methods: PEG and GSH-PEG hydrogels were prepared using ultraviolet light (UV)-polymerization. Hydrogel loading and release of selected drug candidates was examined using UV-visible spectrometry. Therapeutic molecules and GST-fusion protein loading was examined using UV-visible and fluorescent spectrometry. Efficacy of released meropenem was assessed against meropenem-sensitive and -resistant P. aeruginosa in an agar diffusion bioassay.
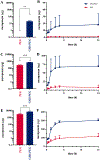
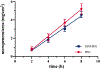
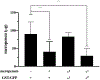
Results: For all tested agents, GSH-PEG hydrogels demonstrated time-dependent loading whereas PEG hydrogels did not. GSH-PEG hydrogels released meropenem over 24 h. Co-loading of biologic and traditional therapeutics into a single vehicle was successfully demonstrated. Meropenem-loaded GSH-PEG hydrogels inhibited the growth of meropenem-sensitive and resistant P. aeruginosa isolates.
Conclusion: GSH ligands within GSH-PEG hydrogels allow loading and effective delivery of charged therapeutic agents, in addition to biologic therapeutics.
Keywords: antibiotic delivery; biomaterials; glutathione; hydrogel; infection; protein delivery.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Competing Interest and/or Conflicts of Interests
E.W. serves on the speaker’s bureau for Melinta Therapeutics, Astellas Pharma, and Allergan Plc., and on the advisory board for GenMark Diagnostics and Shionogi. All other authors certify no potential conflicts of interest.
Figures
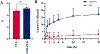
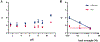
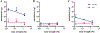
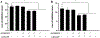
Similar articles
-
Assessment of antibiotic release and antibacterial efficacy from pendant glutathione hydrogels using ex vivo porcine skin.J Control Release. 2024 Jan;365:936-949. doi: 10.1016/j.jconrel.2023.12.008. Epub 2023 Dec 19. J Control Release. 2024. PMID: 38070603 Free PMC article.
-
Phenylboronic Acid Functionalized Polycarbonate Hydrogels for Controlled Release of Polymyxin B in Pseudomonas Aeruginosa Infected Burn Wounds.Adv Healthc Mater. 2018 Jul;7(13):e1701388. doi: 10.1002/adhm.201701388. Epub 2018 Mar 6. Adv Healthc Mater. 2018. PMID: 29508561
-
Preparation and characterization of infection-resistant antibiotics-releasing hydrogels rods of poly[hydroxyethyl methacrylate-co-(poly(ethylene glycol)-methacrylate]: biomedical application in a novel rabbit penile prosthesis model.J Biomed Mater Res B Appl Biomater. 2008 Jul;86(1):18-28. doi: 10.1002/jbm.b.30983. J Biomed Mater Res B Appl Biomater. 2008. PMID: 18098187
-
Evaluation of studies on extended versus standard infusion of beta-lactam antibiotics.Am J Health Syst Pharm. 2019 Sep 3;76(18):1383-1394. doi: 10.1093/ajhp/zxz154. Am J Health Syst Pharm. 2019. PMID: 31505562 Review.
-
PEG hydrogels for the controlled release of biomolecules in regenerative medicine.Pharm Res. 2009 Mar;26(3):631-43. doi: 10.1007/s11095-008-9801-2. Epub 2008 Dec 18. Pharm Res. 2009. PMID: 19089601 Free PMC article. Review.
Cited by
-
Assessment of antibiotic release and antibacterial efficacy from pendant glutathione hydrogels using ex vivo porcine skin.J Control Release. 2024 Jan;365:936-949. doi: 10.1016/j.jconrel.2023.12.008. Epub 2023 Dec 19. J Control Release. 2024. PMID: 38070603 Free PMC article.
-
Hybrid Hydrogels for Neomycin Delivery: Synergistic Effects of Natural/Synthetic Polymers and Proteins.Polymers (Basel). 2023 Jan 26;15(3):630. doi: 10.3390/polym15030630. Polymers (Basel). 2023. PMID: 36771933 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

